STERILE WATER- water injection, solution
Sterile Water by
Drug Labeling and Warnings
Sterile Water by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Company, Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Sterile Water for Injection, USP, is sterile, nonpyrogenic, distilled water in a single dose container for intravenous administration after addition of a suitable solute. It may also be used as a dispensing container for diluent use. No antimicrobial or other substance has been added. The pH is 5.5 (5.0 to 7.0). The osmolarity is 0.
The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
-
ADVERSE REACTIONS
The administration of a suitable admixture of prescribed additives may be associated with adverse reactions because of the solution or the technique of administration including febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
-
DOSAGE AND ADMINISTRATION
Following suitable admixture of prescribed additives, the dosage is usually dependent upon the age, weight and clinical condition of the patient as well as laboratory determinations. See directions accompanying additive drug.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Injections in VIAFLEX plastic containers are intended for intravenous administration using sterile equipment.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. If, in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. Do not store an unused portion of Sterile Water for Injection, USP. Mix thoroughly when additives have been introduced. Do not store solutions containing additives.
-
HOW SUPPLIED
Sterile Water for Injection, USP is supplied in VIAFLEX plastic containers as follows:
1000 mL
2B0304
NDC: 0338-0013-04
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
-
DIRECTIONS FOR USE OF VIAFLEX PLASTIC CONTAINER
Warning: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
To Open
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the port outlet protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired. See following directions.
Preparation for Administration After Rendering Isotonic
- 1. Suspend container from eyelet support.
- 2. Remove plastic protector from outlet port at bottom of container.
- 3. Attach administration set. Refer to complete directions accompanying set.
Warning: Additives may be incompatible.
To add medication before administration
- 1. Prepare medication site.
- 2. Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
- 3. Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To add medication during administration
- 1. Close clamp on the set.
- 2. Prepare medication site.
- 3. Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
- 4. Remove container from IV pole and/or turn to an upright position.
- 5. Evacuate both ports by squeezing them while container is in the upright position.
- 6. Mix solution and medication thoroughly.
- 7. Return container to in use position and continue administration.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
07 19 73 675
Rev. September 2014
Baxter, Viaflex, and PL 146 are trademarks of Baxter International Inc.
-
PACKAGE LABEL.PRINCIPLE DISPLAY PANEL
Sterile Water Container Label
LOT EXP
2B0304
NDC: 0338-0013-04
STERILE WATER
STERILE WATER FOR INJECTION USP
FOR DRUG DILUENT USE ONLY
1000mL
NO ANTIMICROBIAL OR OTHER SUBSTANCE HAS BEEN ADDED
pH 5.5 (5.0 TO 7.0) STERILE NONPYROGENIC SINGLE
DOSE CONTAINER ADMINISTER INTRAVENOUSLY ONLY AFTER
RENDERING APPROXIMATELY ISOTONIC WITH SUITABLE SOLUTE
ADDITIVES MAY BE INCOMPATIBLE CONSULT WITH PHARMACIST
IF AVAILABLE WHEN INTRODUCING ADDITIVES USE ASEPTIC
TECHNIQUE MIX THOROUGHLY DO NO STORE DOSAGE
INTRAVENOUSLY AS DIRECTED BY A PHYSICIAN SEE
DIRECTIONS CAUTIONS SQUEEZE AND INSPECT INNER BAG
WHICH MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE
FOUND MUST NOT BE USED IN SERIES CONNECTIONS DO NOT
USE UNLESS SOLUTION IS CLEAR RX ONLY STORE UNIT IN
MOISTURE BARRIER OVERWRAP AT ROOM TEMPERATURE (25°C)
UNTIL READY TO USE AVOID EXCESSIVE HEAT SEE INSERT
VIAFLEX PLUS CONTAINER PL 146 PLASTIC
BAXTER VIAFLEX AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INC
FOR PRODUCT INFORMATION 1-800-933-0303
BAXTER
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN THE USA
Sterile Water Carton Label
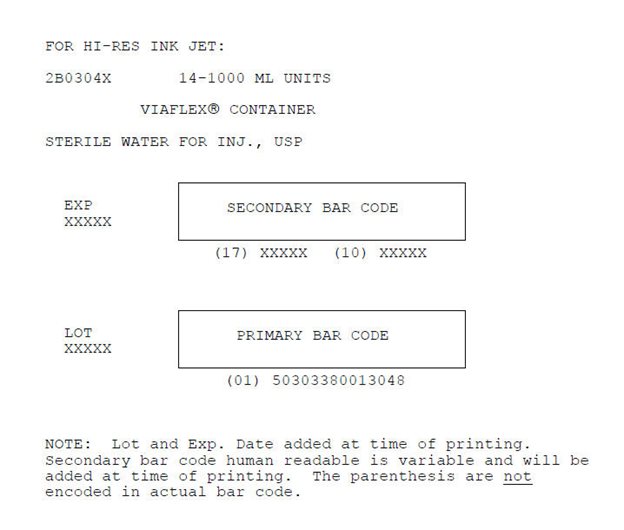
2B0304X 14-1000 ML UNITS
VIAFLEX® CONTAINER
STERILE WATER FOR INJ., USP
EXP
XXXXX
SECONDARY BAR CODE
(17) XXXXX (10) XXXXX
LOT
XXXXX
PRIMARY BAR CODE
(01) 50303380013048
NOTE: Lot and Exp. Date added at time of printing.
Secondary bar code human readable is variable and will be
added at time of printing. The parenthesis are not
encoded in actual bar code.
-
INGREDIENTS AND APPEARANCE
STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0013 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 100 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0013-04 1000 mL in 1 BAG; Type 0: Not a Combination Product 06/30/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018632 06/30/1982 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 API MANUFACTURE(0338-0013) , ANALYSIS(0338-0013) , LABEL(0338-0013) , MANUFACTURE(0338-0013) , PACK(0338-0013) , STERILIZE(0338-0013) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.