3M AVAGARD- chlorhexidine gluconate and alcohol lotion
3M Avagard by
Drug Labeling and Warnings
3M Avagard by is a Otc medication manufactured, distributed, or labeled by 3M Health Care . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
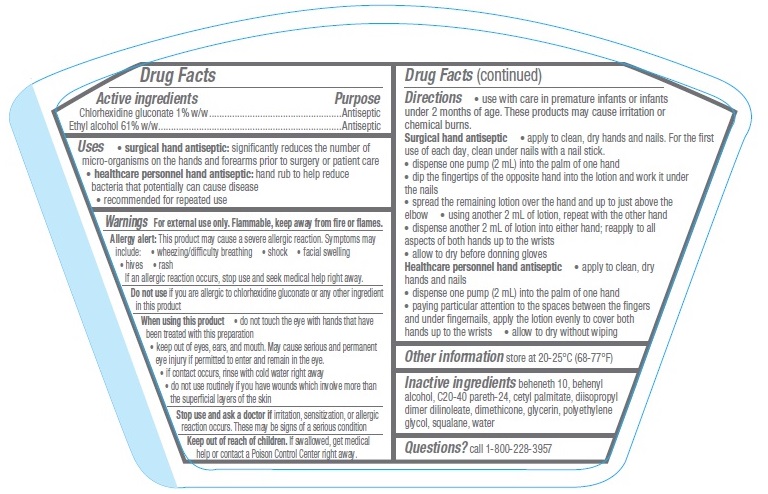
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only. Flammable, keep away from fire or flame.
Allergy alert: This product may cause a severe allergic reaction. Symptoms may include:- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- do not touch the eye with hands that have been treated with this preparation
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye.
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
-
Directions
- Use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand antiseptic
- apply to clean, dry hands and nails. For the first use of each day, clean under nails with a nail stick.
- dispense one pump (2 mL) into the palm of one hand
- dip the fingertips of the opposite hand into the lotion and work it under the nails
- spread the remaining lotion over the hand and up to just above the elbow
- using another 2 mL of lotion, repeat with the other hand
- dispense another 2 mL of lotion into either hand; reapply to all aspects of both hands up to the wrists
- allow to dry before donning gloves
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 500mL Bottle Label
3M
NDC: 17518-051-01
Avagard™
(Chlorhexidine Gluconate 1% Solution and Ethyl Alcohol 61% w/w)
Surgical and Healthcare Personnel Hand Antiseptic with Moisturizers
Peel to read Drug Facts Panel before use
Made in U.S.A. for 3M Health Care, 2510 Conway Ave., St. Paul, MN 55144
1-800-228-3957 www.3M.com/infectionprevention © 2017, 3M. All rights reserved.
The shape and colors of the bottle and wall bracket are trademarks of 3M.
Patent: 3M.com/Patents MAL10110039XC
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
LOT 0000000000
EXP 0000-00-00
16 fl oz 500 mL
REF 9200

-
INGREDIENTS AND APPEARANCE
3M AVAGARD
chlorhexidine gluconate and alcohol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17518-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Chlorhexidine Gluconate (UNII: MOR84MUD8E) (Chlorhexidine - UNII:R4KO0DY52L) Chlorhexidine Gluconate 8.3 mg in 1 mL Alcohol (UNII: 3K9958V90M) (Alcohol - UNII:3K9958V90M) Alcohol 506.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength beheneth-10 (UNII: 313S43DM16) docosanol (UNII: 9G1OE216XY) cetyl palmitate (UNII: 5ZA2S6B08X) dimethicone (UNII: 92RU3N3Y1O) glycerin (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) squalane (UNII: GW89575KF9) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17518-051-01 8 in 1 CASE 06/14/2001 1 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 17518-051-04 4 in 1 CASE 06/14/2001 2 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021074 06/14/2001 Labeler - 3M Health Care (006173082)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.