GEL-KAM MINT- stannous fluoride gel, dentifrice GEL-KAM FRUIT AND BERRY- stannous fluoride gel, dentifrice
Gel-Kam Fruit and Berry by
Drug Labeling and Warnings
Gel-Kam Fruit and Berry by is a Otc medication manufactured, distributed, or labeled by Colgate Oral Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
- Uses
- Warnings
-
Directions
- adults and children 12 years of age or older: use after regular brushing and flossing
- shake excess water from your toothbrush and cover bristles with gel
- brush thoroughly
- keep on your teeth for one minute and then spit out
- do not swallow
- do not rinse, eat or drink for 30 minutes
- use once a day for cavity protection
- use twice a day (morning and evening) for sensitivity relief or as recommended by a dentist or physician
- make sure all sensitive areas are covered by using a toothbrush or a cotton swab
- supervise children until capable of using without supervision
- children under 12 years of age: consult a dentist or physician
-
Other information
- this is a fluoride preventive treatment gel, not a toothpaste. Read directions carefully before use
- this product may produce surface staining of the teeth Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist
- do not use if embossed seal on tube is broken or missing
- to open tube, remove cap and pierce foil seal with point of cap
- keep tightly closed when not in use
- store at controlled room temperature 68-77°F (20-25°C)
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 122 g Tube Carton - Mint
NDC: 0126-0192-93
Colgate®
GelKam®
0.4% Stable Stannous Fluoride
PREVENTATIVE TREATMENT GEL
For Cavity Protection and Sensitive Teeth
MINTNET WT 4.3 OZ (122 g)
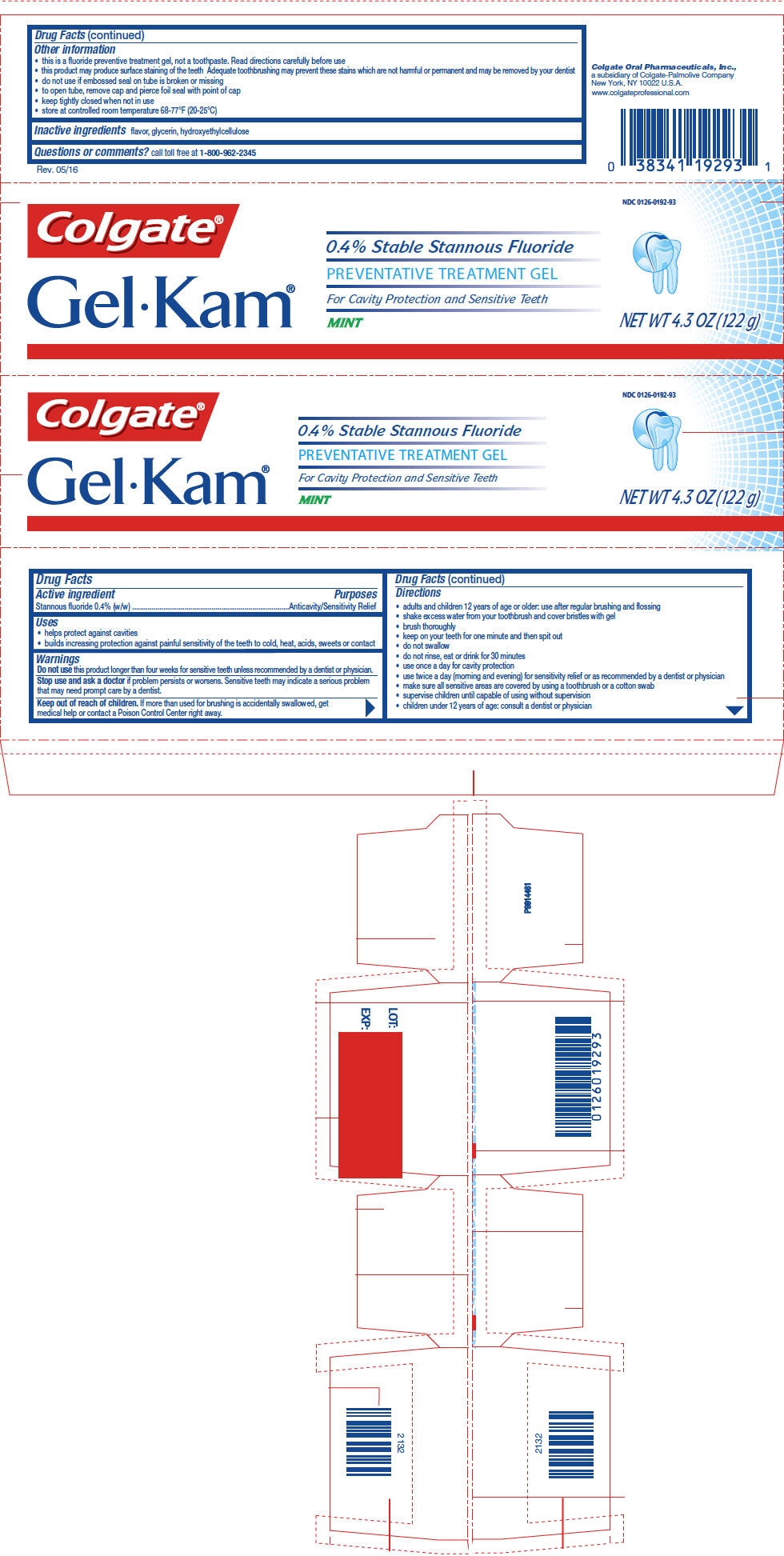
-
PRINCIPAL DISPLAY PANEL - 122 g Tube Carton - Fruit and Berry
NDC: 0126-0165-93
Colgate®
GelKam®
0.4% Stable Stannous Fluoride
PREVENTATIVE TREATMENT GEL
For Cavity Protection and Sensitive Teeth
FRUIT & BERRYNET WT 4.3 OZ (122 g)
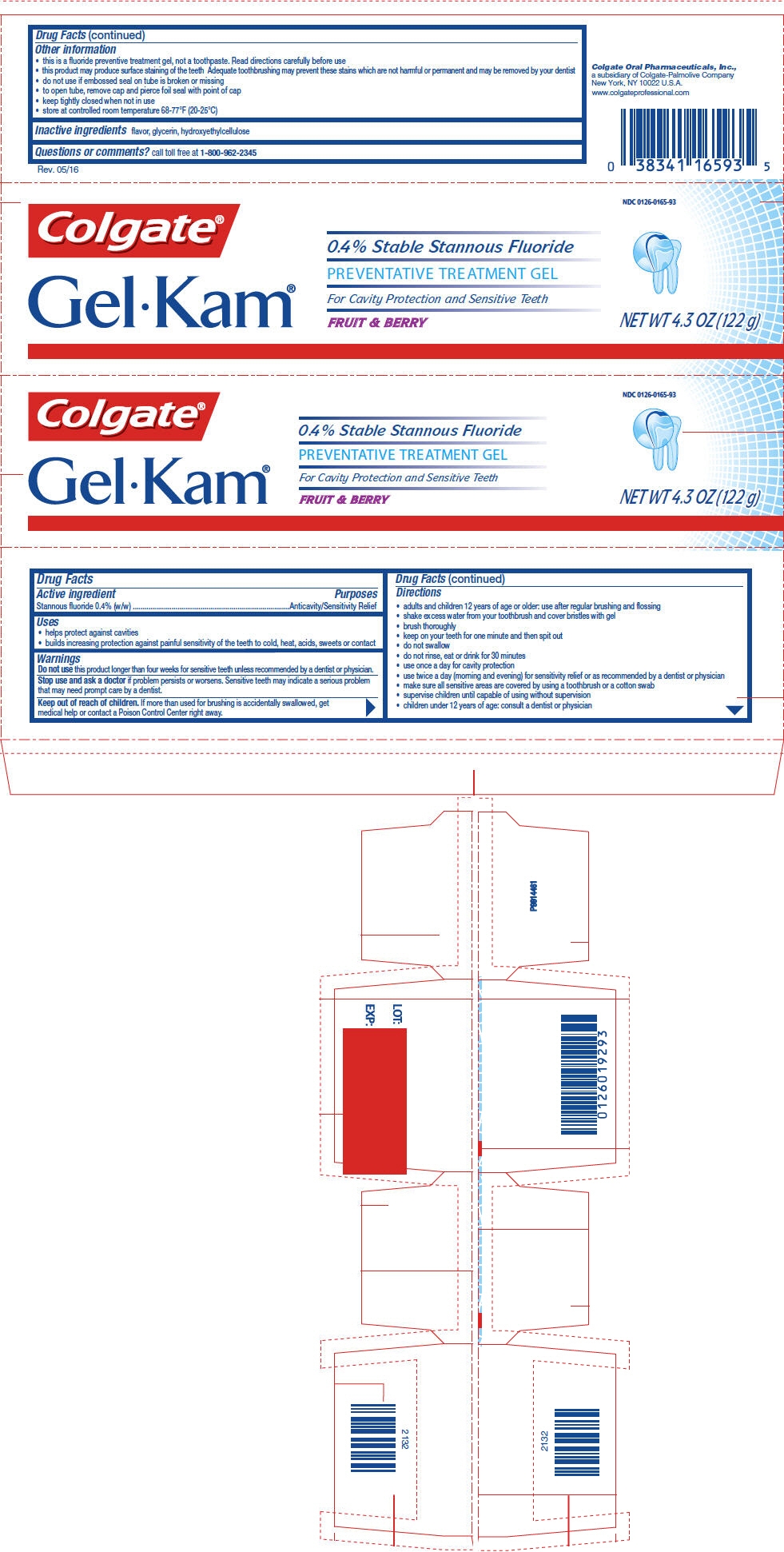
-
INGREDIENTS AND APPEARANCE
GEL-KAM MINT
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0126-0192 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0126-0192-93 1 in 1 CARTON 01/01/1990 1 122 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 01/01/1990 GEL-KAM FRUIT AND BERRY
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0126-0165 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0126-0165-93 1 in 1 CARTON 01/01/1990 1 122 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 01/01/1990 Labeler - Colgate Oral Pharmaceuticals, Inc. (968801118)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.