NITECLEAR COLD AND FLU RELIEF
NITECLEAR COLD AND FLU RELIEF by
Drug Labeling and Warnings
NITECLEAR COLD AND FLU RELIEF by is a Otc medication manufactured, distributed, or labeled by GM Pharmaceuticals, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NITECLEAR COLD AND FLU RELIEF- choline salicylate, diphenhydramine hci, phenylephrine hcl solution
GM Pharmaceuticals, INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NITECLEAR COLD AND FLU RELIEF
NiteClear® Cold & Flu Relief
(choline salicylate, diphenhydramine hcI, phenylephrine hcl), solution
GM Pharmaceuticals, Inc.
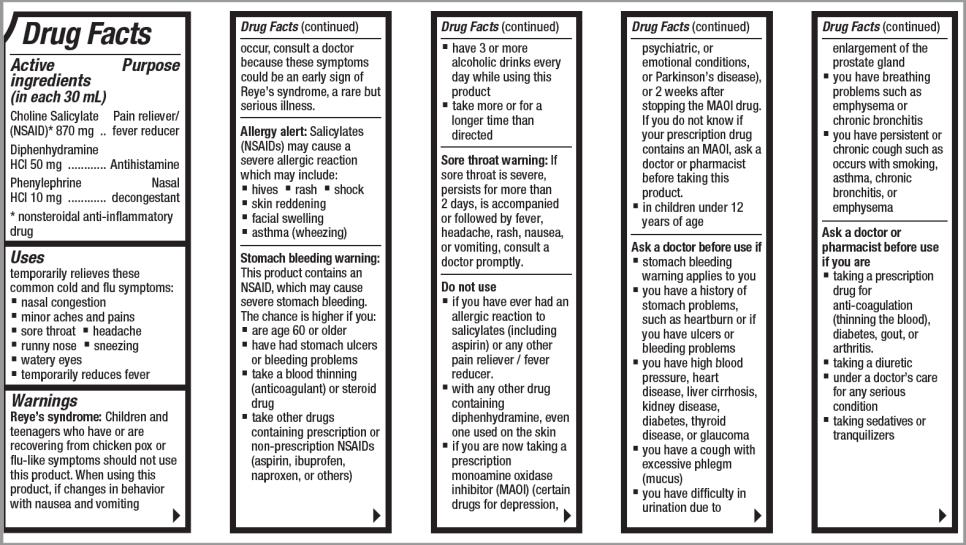
Drug Facts
Active ingredients (in each 30 mL)
Choline Salicylate (NSAID)* 870 mg
Diphenhydramine HCI 50mg
Phenylephrine HCl 10 mg
Purpose
Pain reliever/fever reducer
Antihistamine
Nasal decongestant
nonsteroidal anti-inflammatory drug*
Uses
▪temporarily relieves these common cold and flu symptoms:
▪ nasal congestion
▪ minor aches and pains
▪ runny nose
▪ sneezing
▪ watery eyes
▪ sore throat
▪ headache
▪ temporarily reduces fever
Warnings
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert Salicylates (NSAIDs) may cause a severe allergic reaction which may include:
▪ hives
▪ rash
▪ shock
▪ skin reddening
▪ facial swelling
▪ asthma (wheezing)
Stomach bleeding warnings
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
▪ are age 60 or older
▪ have had stomach ulcers or bleeding problems
▪ take a blood thinning (anticoagulant) or steroid drug
▪ take other drugs containing prescription or non-prescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
▪ have 3 or more alcoholic drinks every day while using this product
▪ take more or for a longer time than directed
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or
followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
▪ if you have ever had an allergic reaction to salicylates (including aspirin) or any other pain reliever / fever reducer.
▪ with any other drug containing diphenhydramine, even one used on the skin
▪ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
▪ in children under 12 years of age
Ask a doctor before use if
▪ stomach bleeding warning applies to you
▪ you have a history of stomach problems, such as heartburn or if you have ulcers or bleeding problems
▪ you have high blood pressure, heart disease, liver cirrhosis, kidney disease, diabetes, thyroid disease, or glaucoma
▪ you have a cough with excessive phlegm (mucus)
▪ you have difficulty in urination due to enlargement of the prostate gland
▪ you have breathing problems such as emphysema or chronic bronchitis
▪ you have persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are
▪ taking a prescription drug for anticoagulation (thinning the blood), diabetes, gout, or arthritis.
▪ taking a diuretic
▪ under a doctor’s care for any serious condition
▪ taking sedatives or tranquilizers
When using this product
▪ do not use more than directed
▪ excitability may occur, especially in children
▪ marked drowsiness may occur
▪ alcohol, sedatives, and tranquilizers may increase drowsiness
▪ avoid alcoholic drinks
▪ be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
▪ an allergic reaction occurs. Seek medical help right away.
▪ pain, cough, or nasal congestion gets worse or lasts more than 7 days
▪ fever gets worse or lasts more than 3 days
▪ redness or swelling is present
▪ new symptoms occur
▪ ringing in the ears or a loss of hearing occurs
▪ cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
▪ nervousness, dizziness, or sleeplessness occurs
▪ you experience any of the following signs of stomach bleeding
▪ feel faint
▪ vomit blood
▪ have bloody or black stools
▪ have stomach pain that does not get better
Directions
▪ do not exceed recommended dosage.
▪ do not take more than 6 doses in any 24-hour period.
▪ use enclosed dose cup
▪ keep dosage cup with product
▪ mL= milliliter
▪ adults and children 12 years and over: 30 mL every 4 hours
▪ children under 12: do not use
Other information
▪ each 30 mL contains: Sodium 16 mg
▪ store at room temperature 68o-86oF (20o-30oC)
| NITECLEAR COLD AND FLU RELIEF
choline salicylate, diphenhydramine hci, phenylephrine hcl solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GM Pharmaceuticals, INC (793000860) |