Fexofenadine HCl and Pseudoephedrine HCl
Fexofenadine HCl and Pseudoephedrine HCl by
Drug Labeling and Warnings
Fexofenadine HCl and Pseudoephedrine HCl by is a Prescription medication manufactured, distributed, or labeled by Dr.Reddy's Laboratories Limited, Dr.Reddy's Laboratories Limited-FTO3. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FEXOFENADINE HCL AND PSEUDOEPHEDRINE HCL - fexofenadine hcl and pseudoephedrine hcl tablet, extended release
Dr.Reddy's Laboratories Limited
----------
Fexofenadine HCl and Pseudoephedrine HCl
DESCRIPTION
Fexofenadine HCl 180 mg and Pseudoephedrine HCl 240 mg Extended-Release Tablets USP(24 Hour Formulation) for oral administration contain 180 mg fexofenadine hydrochloride USP for immediate release and 240 mg pseudoephedrine hydrochloride USP for extended release. Tablets also contain as excipients: acetyltributyl citrate, colloidal silicon dioxide, copovidone, croscarmellose sodium, ethylcellulose, hydrogenated vegetable oil, hypromellose, microcrystalline cellulose, polyethylene glycol, sodium stearyl fumarate, talc and titanium dioxide.
Fexofenadine hydrochloride USP, one of the active ingredients of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets USP (24 hour formulation), is a histamine H1-receptor antagonist with the chemical name (±)-4-[1-hydroxy-4-[4- (hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride and the following chemical structure:
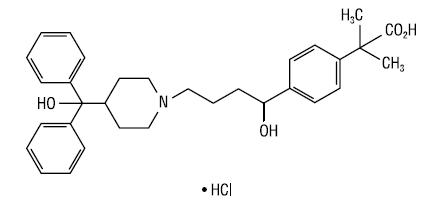
The molecular weight is 538.13 and the empirical formula is C32H39NO4HCl. Fexofenadine hydrochloride is a white to off-white powder. It is soluble in methanol. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH.
Pseudoephedrine hydrochloride USP, the other active ingredient of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets USP (24 hour formulation), is an adrenergic (vasoconstrictor) agent with the chemical name [S-(R*,R*)]-α-[1- (methylamino)ethyl]-benzenemethanol hydrochloride and the following chemical structure:
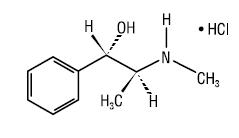
The molecular weight is 201.70 and the molecular formula is C10H15NOHCl. Pseudoephedrine hydrochloride occurs as fine, white to off-white crystals or powder, having a faint characteristic odor. It is very soluble in water, freely soluble in alcohol; and sparingly soluble in chloroform.
CLINICAL PHARMACOLOGY
Mechanism of Action
Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine release from peritoneal mast cells in rats. In laboratory animals, no anticholinergic or alpha1-adrenergic-receptor blocking effects were observed. Moreover, no sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier.
Pseudoephedrine hydrochloride is an orally active sympathomimetic amine and exerts a decongestant action on the nasal mucosa. Pseudoephedrine hydrochloride is recognized as an effective agent for the relief of nasal congestion due to allergic rhinitis. Pseudoephedrine produces peripheral effects similar to those of ephedrine and central effects similar to, but less intense than, amphetamines. It has the potential for excitatory side effects.
Pharmacokinetics
The pharmacokinetics of fexofenadine hydrochloride in subjects with seasonal allergic rhinitis were similar to those in healthy volunteers.
Absorption:
Fexofenadine hydrochloride and pseudoephedrine hydrochloride administered as fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) are absorbed at a similar rate and are equally available under single-dose and steady-state conditions as the separate administration of the components. Coadministration of fexofenadine and pseudoephedrine does not significantly affect the bioavailability of either component. The administration of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) 30 minutes or 1.5 hour after a high-fat meal decreased the bioavailability of fexofenadine by approximately 50% (AUC 42% and Cmax 54%). Pseudoephedrine pharmacokinetics were unaffected when coadministered with a high-fat meal. Therefore, fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) should be taken on an empty stomach with water (see DOSAGE AND ADMINISTRATION).
A pharmacokinetic study following single and multiple oral doses over 7 days of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) in 66 healthy volunteers showed that fexofenadine, the immediate release component of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation), was rapidly absorbed with mean maximum plasma concentrations of 634 ng/mL and 674 ng/mL after single and multiple doses, respectively. The median time to maximum concentration of fexofenadine was 1.8-2.0 hours post-dose. In the same study, the mean maximum plasma concentrations of pseudoephedrine, the extended-release component of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation), were 394 ng/mL and 495 ng/mL after single and multiple doses, respectively, with median time to maximum concentration of 12 hours post-dose. Pseudoephedrine concentrations at the end of the dosing interval (mean: 172 ng/mL) at steady state were equivalent to those observed from a comparator pseudoephedrine hydrochloride 240 mg tablet.
Distribution
Fexofenadine hydrochloride is 60% to 70% bound to plasma proteins, primarily albumin and α1-acid glycoprotein. The protein binding of pseudoephedrine in humans is not known. Pseudoephedrine hydrochloride is extensively distributed into extravascular sites (apparent volume of distribution between 2.6 and 3.5 L/kg).
Metabolism
Approximately 5% of the total dose of fexofenadine hydrochloride and less than 1% of the total oral dose of pseudoephedrine hydrochloride were eliminated by hepatic metabolism.
Elimination
The mean terminal elimination half-life of fexofenadine was 14.6 hours following administration of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) in healthy volunteers, which is consistent with observations from separate administration. Human mass balance studies documented a recovery of approximately 80% and 11% of the [14C]-fexofenadine hydrochloride dose in the feces and urine, respectively. Because the absolute bioavailability of fexofenadine hydrochloride has not been established, it is unknown if the fecal component is primarily unabsorbed drug or the result of biliary excretion. The mean terminal half-life of pseudoephedrine was 7 hours following single-dose administration of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation).
Pseudoephedrine has been shown to have a mean elimination half-life of 4-6 hours which is dependent on urine pH. The elimination half-life is decreased at urine pH lower than 6 and may be increased at urine pH higher than 8.
Special Populations
Pharmacokinetics in special populations (for renal, hepatic impairment, and age), obtained after a single dose of 80 mg fexofenadine hydrochloride, were compared to those from healthy volunteers in a separate study of similar design.
Effect of Age.
In older subjects (≥65 years old), peak plasma levels of fexofenadine were 99% greater than those observed in younger subjects (<65 years old). Mean fexofenadine elimination half-lives were similar to those observed in younger subjects.
Renally Impaired.
In subjects with mild (creatinine clearance 41-80 mL/min) to severe (creatinine clearance 11-40 mL/min) renal impairment, peak plasma levels of fexofenadine were 87% and 111% greater, respectively, and mean elimination half-lives were 59% and 72% longer, respectively, than observed in healthy volunteers. Peak plasma levels in subjects on dialysis (creatinine clearance ≤10 mL/min) were 82% greater and half-life was 31% longer than observed in healthy volunteers. No data are available on the pharmacokinetics of pseudoephedrine in renally impaired subjects. However, most of the oral dose of pseudoephedrine hydrochloride (43- 96%) is excreted unchanged in the urine. A decrease in renal function is, therefore, likely to decrease the clearance of pseudoephedrine significantly, thus prolonging the half-life and resulting in accumulation. (See PRECAUTIONSand DOSAGE AND ADMINISTRATION.)
Hepatically Impaired. The pharmacokinetics of fexofenadine hydrochloride in subjects with hepatic disease did not differ substantially from that observed in healthy volunteers. The effect on pseudoephedrine pharmacokinetics is unknown.
Effect of Gender. Across several trials, no clinically significant gender-related differences were observed in the pharmacokinetics of fexofenadine hydrochloride.
PharmacodynamicsWheal and Flare. Human histamine skin wheal and flare studies following single and twice daily doses of 20 mg and 40 mg fexofenadine hydrochloride demonstrated that the drug exhibits an antihistamine effect by 1 hour, achieves maximum effect at 2-3 hours, and an effect is still seen at 12 hours. There was no evidence of tolerance to these effects after 28 days of dosing. The clinical significance of these observations is unknown.
Effects on QTc. In dogs, (30 mg/kg/orally twice daily for 5 days) and rabbits (10 mg/kg, intravenously over 1 hour) fexofenadine hydrochloride did not prolong QTc at plasma concentrations that were at least 7 and 15 times, respectively, the therapeutic plasma concentrations in man (based on a 180 mg once daily fexofenadine hydrochloride dose when administered as fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation)). No effect was observed on calcium channel current, delayed K+ channel current, or action potential duration in guinea pig myocytes, Na+ current in rat neonatal myocytes, or on the delayed rectifier K+ channel cloned from human heart at concentrations up to 1 x 10-5 M of fexofenadine. This concentration was at least 8 times the therapeutic plasma concentration in man (based on a 180 mg once daily fexofenadine hydrochloride dose).
No statistically significant increase in mean QTc interval compared to placebo was observed in 714 subjects with seasonal allergic rhinitis given fexofenadine hydrochloride capsules in doses of 60 mg to 240 mg twice daily for 2 weeks or in 40 healthy volunteers given fexofenadine hydrochloride as an oral solution at doses up to 400 mg twice daily for 6 days.
A 1-year study designed to evaluate safety and tolerability of 240 mg of fexofenadine hydrochloride (n=240) compared to placebo (n=237) in healthy volunteers, did not reveal a statistically significant increase in the mean QTc interval for the fexofenadine hydrochloride treated group when evaluated pretreatment and after 1, 2, 3, 6, 9, and 12 months of treatment. Administration of the 60 mg fexofenadine hydrochloride/120 mg pseudoephedrine hydrochloride combination tablet for approximately 2 weeks to 213 subjects with seasonal allergic rhinitis demonstrated no statistically significant increase in the mean QTc interval compared to fexofenadine hydrochloride administered alone (60 mg twice daily, n=215), or compared to pseudoephedrine hydrochloride (120 mg twice daily, n=215) administered alone.
CLINICAL TRIALS
Clinical StudiesClinical efficacy and safety studies were not conducted with fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation). The effectiveness of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) for the treatment of seasonal allergic rhinitis is based on an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride 180 mg and the nasal decongestant properties of pseudoephedrine hydrochloride.
In one 2-week, multicenter, randomized, double-blind clinical trial in subjects 12 to 65 years of age with seasonal allergic rhinitis (n=863), fexofenadine hydrochloride 180 mg once daily significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes) compared to placebo. Although the number of subjects in some of the subgroups was small, there were no significant differences in the effect of fexofenadine hydrochloride across subgroups of subjects defined by gender, age, and race.
INDICATIONS AND USAGE
Fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) are indicated for the relief of symptoms associated with seasonal allergic rhinitis in adults and children 12 years of age and older. Symptoms treated effectively include sneezing, rhinorrhea, itchy nose/palate/ and/or throat, itchy/watery/red eyes, and nasal congestion.
Fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) should be administered when both the antihistaminic properties of fexofenadine hydrochloride and the nasal decongestant properties of pseudoephedrine hydrochloride are desired (see CLINICAL PHARMACOLOGY).
CONTRAINDICATIONS
Fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) are contraindicated in patients with known hypersensitivity to any of its ingredients.
Due to its pseudoephedrine component, fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) are contraindicated in patients with narrow-angle glaucoma or urinary retention, and in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment (see Drug Interactions section). It is also contraindicated in patients with severe hypertension, or severe coronary artery disease, and in those who have shown idiosyncrasy to its components, to adrenergic agents, or to other drugs of similar chemical structures. Manifestations of patient idiosyncrasy to adrenergic agents include: insomnia, dizziness, weakness, tremor, or arrhythmias.
WARNINGS
Sympathomimetic amines should be used with caution in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, renal impairment, or prostatic hypertrophy (see CONTRAINDICATIONS). Sympathomimetic amines may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension.
PRECAUTIONS
General
Because fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) are a once-daily, fixed-dose combination that cannot be titrated and renal insufficiency increases the bioavailability and prolongs the half-life of fexofenadine hydrochloride and pseudoephedrine hydrochloride, fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) should generally be avoided in patients with renal insufficiency (see CLINICAL PHARMACOLOGY, and DOSAGE AND ADMINISTRATION).
Information for Patients
Patients taking fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) should receive the following information: fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg (24 hour formulation) extended-release tablets are prescribed for the relief of symptoms of seasonal allergic rhinitis. Patients should be instructed to take fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) only as prescribed. Do not exceed the recommended dose. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult the doctor.Patients should also be advised against the concurrent use of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) with over-the-counter antihistamines and decongestants.
The product should not be used by patients who are hypersensitive to it or to any of its ingredients. Due to its pseudoephedrine component, this product should not be used by patients with narrow-angle glaucoma, urinary retention, or by patients receiving a monoamine oxidase (MAO) inhibitor or within 14 days of stopping use of MAO inhibitor. It also should not be used by patients with severe hypertension or severe coronary artery disease.
Patients should be told that this product should be used in pregnancy or lactation only if the potential benefit justifies the potential risk to the fetus or nursing infant. Patients should be advised to take the tablet on an empty stomach with water. Patients should be directed to swallow the tablet whole. Patients should be cautioned not to break or chew the tablet. Patients should also be instructed to store the medication in a tightly closed container in a cool, dry place, away from children.
Drug Interactions
Fexofenadine hydrochloride and pseudoephedrine hydrochloride do not influence the pharmacokinetics of each other when administered concomitantly.
Fexofenadine has been shown to exhibit minimal (ca. 5%) metabolism. However, coadministration of fexofenadine hydrochloride with either ketoconazole or erythromycin led to increased plasma concentrations of fexofenadine. Fexofenadine had no effect on the pharmacokinetics of either erythromycin or ketoconazole. In 2 separate studies, fexofenadine hydrochloride 120 mg twice daily was co-administered with either erythromycin 500 mg every 8 hours or ketoconazole 400 mg once daily under steady-state conditions to healthy volunteers (n=24, each study). No differences in adverse events or QTc interval were observed when subjects were administered fexofenadine hydrochloride alone or in combination with erythromycin or ketoconazole. The findings of these studies are summarized in the following table:
Effects on steady-state fexofenadine pharmacokineticsafter 7 days of co-administration with fexofenadine hydrochloride120 mg every 12 hours (two times the recommended twice daily dose)in healthy volunteers (n=24)
Concomitant Drug CmaxSS AUCss(0-12h)
(Extent of systemic exposure) (Peak plasma concentration)
Erythromycin +82% +109%
(500 mg every 8 hrs)
Ketoconazole +135% +164%
(400 mg once daily)
The changes in plasma levels were within the range of plasma levels achieved in adequate and well-controlled clinical trials.
The mechanism of these interactions has been evaluated in in vitro, in situ, and in vivo animal models. These studies indicate that ketoconazole or erythromycin co-administration enhances fexofenadine gastrointestinal absorption. This observed increase in the bioavailability of fexofenadine may be due to transport-related effects, such as p-glycoprotein. In vivo animal studies also suggest that in addition to enhancing absorption, ketoconazole decreases fexofenadine gastrointestinal secretion, while erythromycin may also decrease biliary excretion.
Due to the pseudoephedrine component, fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) are contraindicated in patients taking monoamine oxidase inhibitors and for 14 days after stopping use of an MAO inhibitor. Concomitant use with antihypertensive drugs which interfere with sympathetic activity (e.g., methyldopa, mecamylamine, and reserpine) may reduce their antihypertensive effects. Increased ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Care should be taken in the administration of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) concomitantly with other sympathomimetic amines because combined effects on the cardiovascular system may be harmful to the patient (see WARNINGS).
Drug Interactions with Antacids
Administration of 120 mg of fexofenadine hydrochloride (2 x 60 mg capsule) within 15 minutes of an aluminum and magnesium containing antacid (Maalox®) decreased fexofenadine AUC by 41% and Cmax by 43%. Fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) should not be taken closely in time with aluminum and magnesium containing antacids.
Interactions with Fruit Juices
Fruit juices such as grapefruit, orange and apple may reduce the bioavailability and exposure of fexofenadine. This is based on the results from 3 clinical studies using histamine induced skin wheals and flares coupled with population pharmacokinetic analysis. The size of wheal and flare were significantly larger when fexofenadine hydrochloride was administered with either grapefruit or orange juices compared to water. Based on the literature reports, the same effects may be extrapolated to other fruit juices such as apple juice. The clinical significance of these observations is unknown. In addition, based on the population pharmacokinetics analysis of the combined data from grapefruit and orange juices studies with the bioequivalence study data, the bioavailability of fexofenadine was reduced by 36%. Therefore, to maximize the effects of fexofenadine, it is recommended that fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) should be taken with water (see DOSAGE AND ADMINISTRATION).
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no animal or in vitro studies on the combination product fexofenadine hydrochloride and pseudoephedrine hydrochloride to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
The carcinogenic potential and reproductive toxicity of fexofenadine hydrochloride were assessed using terfenadine studies with adequate fexofenadine exposure (area-under-the plasma concentration versus time curve [AUC]). No evidence of carcinogenicity was observed when mice and rats were given daily oral doses up to 150 mg/kg of terfenadine for 18 and 24 months, respectively. In both species, 150 mg/kg of terfenadine produced AUC values of fexofenadine that were approximately 2 and 3 times, respectively, the exposure from the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation).
Two-year feeding studies in rats and mice conducted under the auspices of the National Toxicology Program (NTP) demonstrated no evidence of carcinogenic potential with ephedrine sulfate, a structurally related drug with pharmacological properties similar to pseudoephedrine, at doses up to 10 and 27 mg/kg, respectively (less than the maximum recommended human daily oral dose of pseudoephedrine hydrochloride on a mg/m2 basis).
In in vitro (Bacterial Reverse Mutation, CHO/HGPRT Forward Mutation, and Rat Lymphocyte Chromosomal Aberration assays) and in vivo (Mouse Bone Marrow Micronucleus assay) tests, fexofenadine hydrochloride revealed no evidence of mutagenicity.
Reproduction and fertility studies with terfenadine in rats produced no effect on male or female fertility at oral doses up to 300 mg/kg/day (approximately 3 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs of fexofenadine). However, reduced implants and post-implantation losses were reported at 300 mg/kg. A reduction in implants was also observed at an oral dose of 150 mg/kg/day (approximately 3 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs). In mice, fexofenadine produced no effect on male or female fertility at average dietary doses up to 4438 mg/kg (approximately 10 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs).
Pregnancy: Teratogenic Effects: Pregnancy: Category C.
Terfenadine alone was not teratogenic in rats at oral doses up to 300 mg/kg (approximately 3 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs of fexofenadine) and in rabbits at oral doses up to 300 mg/kg (approximately 25 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs of fexofenadine).
In mice, no adverse effects and no teratogenic effects during gestation were observed with fexofenadine at dietary doses up to 3730 mg/kg (approximately 10 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) based on comparison of the AUCs).
The combination of terfenadine and pseudoephedrine hydrochloride in a ratio of 1:2 by weight was studied in rats and rabbits. In rats, an oral combination dose of 150/300 mg/kg produced reduced fetal weight and delayed ossification with a finding of wavy ribs. The dose of 150 mg/kg of terfenadine in rats produced an AUC value of fexofenadine that was approximately 3 times the AUC of the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation). The dose of 300 mg/kg of pseudoephedrine hydrochloride in rats was approximately 10 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis. In rabbits, an oral combination dose of 100/200 mg/kg produced decreased fetal weight. By extrapolation, the AUC of fexofenadine for 100 mg/kg orally of terfenadine was approximately 8 times the human AUC of the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation). The dose of 200 mg/kg of pseudoephedrine hydrochloride was approximately 15 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis.
There are no adequate and well-controlled studies in pregnant women. Fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects. Dose-related decreases in pup weight gain and survival were observed in rats exposed to an oral dose of 150 mg/kg of terfenadine; this dose produced an AUC of fexofenadine that was approximately 3 times the human AUC of the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation).
Nursing Mothers
It is not known if fexofenadine is excreted in human milk. Because many drugs are excreted in human milk, caution should be used when fexofenadine hydrochloride is administered to a nursing woman. Pseudoephedrine hydrochloride administered alone distributes into breast milk of lactating human females. Pseudoephedrine concentrations in milk are consistently higher than those in plasma. The total amount of drug in milk as judged by AUC is 2 to 3 times greater than the plasma AUC. The fraction of a pseudoephedrine dose excreted in milk is estimated to be 0.4% to 0.7%. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution should be exercised when fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) are administered to nursing women.
Pediatric Use
Safety and effectiveness of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) in children below the age of 12 years have not been established. In addition, the doses of the individual components in fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) exceed the recommended individual doses for pediatric patients under 12 years of age. Fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) is not recommended for pediatric patients under 12 years of age.
Geriatric Use
Clinical studies of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, although the elderly are more likely to have adverse reactions to sympathomimetic amines.
The pseudoephedrine component of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
ADVERSE REACTIONS
Fexofenadine Hydrochloride
In a placebo-controlled clinical study in the United States, which included 570 subjects with seasonal allergic rhinitis aged 12 years and older receiving fexofenadine hydrochloride tablets at doses of 120 or 180 mg once daily, adverse events were similar in fexofenadine hydrochloride and placebo-treated subjects. The following table lists adverse experiences that were reported by greater than 2% of subjects treated with fexofenadine hydrochloride tablets at doses of 180 mg once daily and that were more common with fexofenadine hydrochloride than placebo.
Once daily dosing with fexofenadine hydrochloride
tabletsat rates of greater than 2%
Adverse experience Fexofenadine 180 mg Placebo
once daily (n=293) (n=283)
Headache 10.6% 7.5%
Upper Respiratory 3.2% 3.1%
Tract Infection
Back Pain 2.8% 1.4%
Events that have been reported during controlled clinical trials involving subjects with seasonal allergic rhinitis at incidences less than 1% and similar to placebo and have been rarely reported during postmarketing surveillance include: insomnia, nervousness, and sleep disorders or paroniria. In rare cases, rash, urticaria, pruritus and hypersensitivity reactions with manifestations such as angioedema, chest tightness, dyspnea, flushing and systemic anaphylaxis have been reported.
Pseudoephedrine HydrochloridePseudoephedrine hydrochloride may cause mild CNS stimulation in hypersensitive patients. Nervousness, excitability, restlessness, dizziness, weakness, or insomnia may occur. Headache, drowsiness, tachycardia, palpitation, pressor activity, cardiac arrhythmias and ischemic colitis have been reported. Sympathomimetic drugs have also been associated with other untoward effects such as fear, anxiety, tenseness, tremor, hallucinations, seizures, pallor, respiratory difficulty, dysuria, and cardiovascular collapse.
OVERDOSAGE
Most reports of fexofenadine hydrochloride overdose contain limited information. However, dizziness, drowsiness, and dry mouth have been reported. For the pseudoephedrine hydrochloride component of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation), information on acute overdose is limited to the marketing history of pseudoephedrine hydrochloride. Single doses of fexofenadine hydrochloride up to 800 mg (6 healthy volunteers at this dose level), and doses up to 690 mg twice daily for one month (3 healthy volunteers at this dose level), were administered without the development of clinically significant adverse events.
In large doses, sympathomimetics may give rise to giddiness, headache, nausea, vomiting, sweating, thirst, tachycardia, precordial pain, palpitations, difficulty in micturition, muscular weakness and tenseness, anxiety, restlessness, and insomnia. Many patients can present a toxic psychosis with delusions and hallucinations. Some may develop cardiac arrhythmias, circulatory collapse, convulsions, coma, and respiratory failure.
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Following administration of terfenadine, hemodialysis did not effectively remove fexofenadine, the major active metabolite of terfenadine, from blood (up to 1.7% removed). The effect of hemodialysis on the removal of pseudoephedrine is unknown.
No deaths occurred in mature mice and rats at oral doses of fexofenadine hydrochloride up to 5000 mg/kg (approximately 110 and 230 times, respectively, the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis.) The median oral lethal dose in newborn rats was 438 mg/kg (approximately 20 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis). In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (approximately 300 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis). The oral median lethal dose of pseudoephedrine hydrochloride in rats was 1674 mg/kg (approximately 55 times the maximum recommended human daily oral dose of fexofenadine HCl and pseudoephedrine HCl extended-release tablets (24 hour formulation) on a mg/m2 basis).
DOSAGE AND ADMINISTRATION
The recommended dose of fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) is one tablet once daily administered on an empty stomach with water for adults and children 12 years of age and older fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) should generally be avoided in patients with renal insufficiency. Fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets (24 hour formulation) must be swallowed whole and never crushed or chewed.
HOW SUPPLIED
Fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets USP (24 hour formulation) are off-white to white caplet shaped biconvex film coated tablets debossed with “RDY” on one side and “572” on another side and are supplied in bottles of 30, 60, 100, 500 and unit dose packages of 100 (10 x 10).
Bottles of 30 55111-572-30
Bottles of 60 55111-572-60
Bottles of 100 55111-572-01
Bottles of 500 55111-572-05
Unit dose packages of 100 (10 x 10) 55111-572-78
Store fexofenadine HCl 180 mg and pseudoephedrine HCl 240 mg extended-release tablets USP (24 hour formulation) at 20-25°C (68-77°F). (See USP Controlled Room Temperature.)
Rx Only
Manufactured by
Dr. Reddy’s Laboratories Limited
Bachepalli – 502 325 INDIA
Revised: 0410
| FEXOFENADINE HCL AND PSEUDOEPHEDRINE HCL
fexofenadine hcl and pseudoephedrine hcl tablet, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Dr.Reddy's Laboratories Limited (650562841) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr.Reddy's Laboratories Limited-FTO3 | 918608162 | analysis(55111-572) , manufacture(55111-572) | |

