OSURNIA- florfenicol, terbinafine, betamethasone acetate gel
Osurnia by
Drug Labeling and Warnings
Osurnia by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
- DESCRIPTION:
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
OSURNIA should be administered by a veterinary professional. Wear eye protection when administering Osurnia (see Human Safety Warnings, Precautions, Post-Approval Experience and Animal Safety).
Splatter may occur if the dog shakes its head following administration. Persons near the dog during administration should also take steps to avoid ocular exposure.
- Clean and dry the external ear canal before administering the initial dose of the product.
- Verify the tympanic membrane is intact prior to each administration (see Precautions, Contraindications, Animal Safety and Post-Approval Experience).
- Administer one dose (1 tube) per affected ear(s) and repeat administration in 7 days.
- Open tube by twisting the soft tip. Insert the flexible tip in the affected external ear canal(s) and squeeze entire tube contents into the external ear canal(s). After application, gently massage the base of the ear to allow the gel to penetrate the lower part of the ear canal.
- Restrain dog to minimize post-application head shaking to reduce potential for splatter of product, and accidental eye exposure in people and dogs (see Post-Approval Experience and Animal Safety).
- Do not clean the ear canal for 45 days after the initial administration to allow contact of the gel with the ear canal. Cleaning the ear may affect product effectiveness (see Effectiveness). If alternative otic therapies are required, it is recommended to clean the ear(s) before application.
-
CONTRAINDICATIONS:
Do not use in dogs with known tympanic perforation (see Precautions). Do not use in dogs with a hypersensitivity to florfenicol, terbinafine or corticosteroids.
-
WARNINGS:
Human Safety Warnings:
OSURNIA may cause eye injury and irritation (see Precautions, Post-Approval Experience and Animal Safety).
In case of accidental eye contact, flush thoroughly with water for at least 15 minutes. If symptoms develop, seek medical advice.
Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental ingestion by humans. In case of accidental skin contact, wash area thoroughly with water.
-
PRECAUTIONS:
Wear eye protection when administering OSURNIA and restrain the dog to minimize post-application head shaking. Reducing the potential for splatter of product will help prevent accidental eye exposure in people and dogs and help to prevent ocular injury (see Human Safety Warnings, Post-Approval Experience and Animal Safety).
The use of OSURNIA in dogs with perforated tympanic membranes has not been evaluated. The integrity of the tympanic membrane should be confirmed before administering this product. Reevaluate the dog if hearing loss or signs of vestibular dysfunction are observed during treatment.
Proper patient selection is important when considering the benefits and risks of using OSURNIA. The integrity of the tympanic membrane should be confirmed before administering each dose of product.
Changes to the middle ear, such as ulceration of the mucosal lining, have been associated with OSURNIA administration. (see Animal Safety).
Signs of tympanic membrane rupture, internal ear disease such as head tilt, ataxia, nystagmus, facial paralysis, and keratoconjunctivitis sicca have also been reported (see Post-Approval Experience).
Do not administer orally.
Use of topical otic corticosteroids has been associated with adrenocortical suppression and iatrogenic hyperadrenocorticism in dogs (see Animal Safety).
Use with caution in dogs with impaired hepatic function (see Animal Safety and Adverse Reactions).
The safe use of OSURNIA in dogs used for breeding purposes, during pregnancy, or in lactating bitches, has not been evaluated.
-
ADVERSE REACTIONS:
The following adverse reactions were reported during the course of a US field study for treatment of otitis externa in dogs treated with OSURNIA with 1 tube per affected ear(s) and repeated after 7 days:
Frequency of Adverse Reaction by Treatment Adverse Reaction OSURNIA (n=190) Placebo (n=94) - * Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP). Two dogs with pre-existing elevations in ALP were reported to have an increase in liver enzymes (ALP, ALT and/or AST) at study exit. Subsequent clinical chemistries returned to pre-treatment levels in one dog, while no follow-up was performed for the second dog.
Elevated Alkaline Phosphatase 15 (7.9%) 3 (3.2%) Vomiting 7 (3.7%) 1 (1.1%) Elevated AST, ALT, ALP* 2 (1.1%) 0 (0.0%) Weight loss (>10% body weight) 1 (0.53%) 0 (0.0%) Hearing Decrease/Loss 1 (0.53%) 1 (1.1%) Post-Approval Experience (2020)
The following adverse events are based on post-approval adverse drug experience reporting for OSURNIA. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data.
In humans, accidental exposure leading to corneal ulcers and other ocular injuries such as eye irritation, burning, stinging, and itchiness have been reported to occur when the dog shook its head after application of OSURNIA.
In dogs, the adverse events reported for OSURNIA are presented below in decreasing order of reporting frequency:
Deafness, ear discharge, pinnal irritation and ear pain, emesis, head shaking, internal ear disorder (head tilt and vestibular), ataxia, vocalization, corneal ulcer, keratoconjunctivitis sicca, nystagmus, tympanic rupture, and cranial nerve disorder (facial paralysis).
OSURNIA is not approved for use in cats. The adverse events reported following extra-label use in cats are presented below in decreasing order of reporting frequency:
Ataxia, anorexia, Horner's syndrome (third eyelid prolapse and miosis), internal ear disorder (head tilt and vestibular), anisocoria, lethargy, head shake, emesis, nystagmus, deafness, and tympanic rupture.
-
CONTACT INFORMATION:
To report suspected adverse drug events and/or obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, contact Dechra at 1-866-933-2472
For additional information about adverse drug experience reporting for animal drugs, contact the FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
INFORMATION FOR DOG OWNERS:
Owners should be aware that adverse reactions may occur following administration of OSURNIA and should observe dog for signs such as deafness, ear pain and irritation, vomiting, head shaking, head tilt, incoordination, eye pain and ocular discharge (see Animal Safety and Post-Approval Experience). Owners should be advised to contact their veterinarian if any of the above signs are observed.
Owners should also be informed that splatter may occur if the dog shakes its head following administration of OSURNIA which may lead to ocular exposure. As a result, eye injuries in humans and dogs have been reported including corneal ulcers. Owners should be careful to avoid ocular exposure (see Precautions and Post-Approval Experience).
-
CLINICAL PHARMACOLOGY:
OSURNIA is a fixed combination of three active substances: florfenicol (antibacterial), terbinafine (antifungal) and betamethasone acetate (steroidal anti-inflammatory). Florfenicol is a bacteriostatic antibiotic which acts by inhibiting protein synthesis. Its spectrum of activity includes Gram-positive and Gram-negative bacteria. Terbinafine is an antifungal which selectively inhibits the early synthesis of ergosterol. Betamethasone acetate is a glucocorticosteroid with anti-inflammatory activity.
OSURNIA dissolves in ear wax and is slowly eliminated from the ear mechanically. Ear inflammation can increase the percutaneous absorption of active substances in OSURNIA.
In a laboratory study conducted in healthy dogs (see Animal Safety), low plasma concentrations of florfenicol, terbinafine, and betamethasone acetate were measurable during the first 2-4 days after administration of 1× dose, and during the first 2-7 days after administration of 5× dose. No quantifiable plasma concentrations of any of the three active ingredients were observed in the pre-dose samples of most dogs prior to second and third administrations. Although total and peak exposure in the blood tended to be highly variable between dogs, systemic drug concentrations tended to increase in a less than dose-proportional manner as the administered dose increased from 1× to 5×.
-
MICROBIOLOGY:
The compatibility and additive effect of each of the components in OSURNIA was demonstrated in a component effectiveness and non-interference study. An in vitro study of organisms collected from clinical cases of otitis externa in dogs determined that florfenicol and terbinafine inhibit the growth of bacteria and yeast commonly associated with otitis externa in dogs. No consistent synergistic or antagonistic effect of the two antimicrobials was demonstrated. The addition of betamethasone acetate to the combination did not impair antimicrobial activity to any clinically significant extent.
In a field study (see Effectiveness), the minimum of 10 isolates from successfully treated cases with OSURNIA was met for Staphylococcus pseudintermedius, Malassezia pachydermatis, and Pseudomonas aeruginosa. However, there were only three dogs where P. aeruginosa was the only pathogen cultured and they were all treatment failures. Therefore, OSURNIA may not be effective in treating otitis externa in which P. aeruginosa is the only pathogen present.
-
EFFECTIVENESS:
Effectiveness was evaluated in 235 dogs with otitis externa. The study was a double-masked field study with a placebo control (vehicle without the active ingredients). One hundred and fifty-nine dogs were treated with OSURNIA and seventy-six dogs were treated with the placebo control. All dogs were evaluated for safety. Treatment (1 mL) was administered to the affected ear(s) and repeated 7 days later. Prior to the first administration, the ear(s) were cleaned with saline but not prior to the Day 7 administration. Six clinical signs associated with otitis externa were evaluated: pain, erythema, exudate, swelling, odor and ulceration. Total clinical scores were assigned for a dog based on the severity of each clinical sign on Days 0, 7, 14, 30 and 45. Success was determined by clinical improvement at Day 45. The success rates of the two groups were significantly different (p=0.0094); 64.78% of dogs administered OSURNIA were successfully treated, compared to 43.42% of the dogs in the placebo control group.
-
ANIMAL SAFETY:
In a target animal safety study, 24 mixed breed dogs (4 dogs/sex/group) were aurally administered 0×, 1× (1 mL/ear or 2 mL/dog with repeated administration in 7 days) or 5× (5 mL/ear or 10 mL/dog with repeated administration in 7 days) doses of OSURNIA for a total of 6 administrations in 5 weeks. All dogs remained in good health with normal hearing throughout the study. Decreased weight gain was noted in the 1× and 5× groups compared to the control group. Clinical findings included post-administration ear wetness in 1× and 5× groups and unilateral, transient brown/red discharge from one ear each in two 5× dogs, with erythema in one dog after the 4th application. Local microscopic changes in ears (without clinical effects) included: slight or moderate unilateral vesicle formation within the epithelium of the tympanic membrane in two 1× and four 5× dogs, and unilateral mucosal ulceration in the lining of the middle ear cavity in three 5× dogs. Three 5× dogs had slightly elevated ALT activity, accompanied by minimal or mild microscopic hepatocellular vacuolation (in two dogs). Cortisol response to ACTH stimulation was decreased, but within the normal reference range, in 1× dogs. The 5× dogs had a decrease in serum cortisol levels after ACTH stimulation (below normal reference range) accompanied by decreased adrenal gland and thymic weights with minimal adrenal cortical atrophy and slight (in three dogs) or moderate (in one dog also noted with slightly lower lymphocyte counts) lymphoid depletion of the thymus. The ACTH stimulation test results are consistent with systemic absorption of betamethasone resulting in a likely reversible suppression of the hypothalamic-pituitary-adrenal axis as seen with administration of exogenous corticosteroids.
- STORAGE CONDITIONS:
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
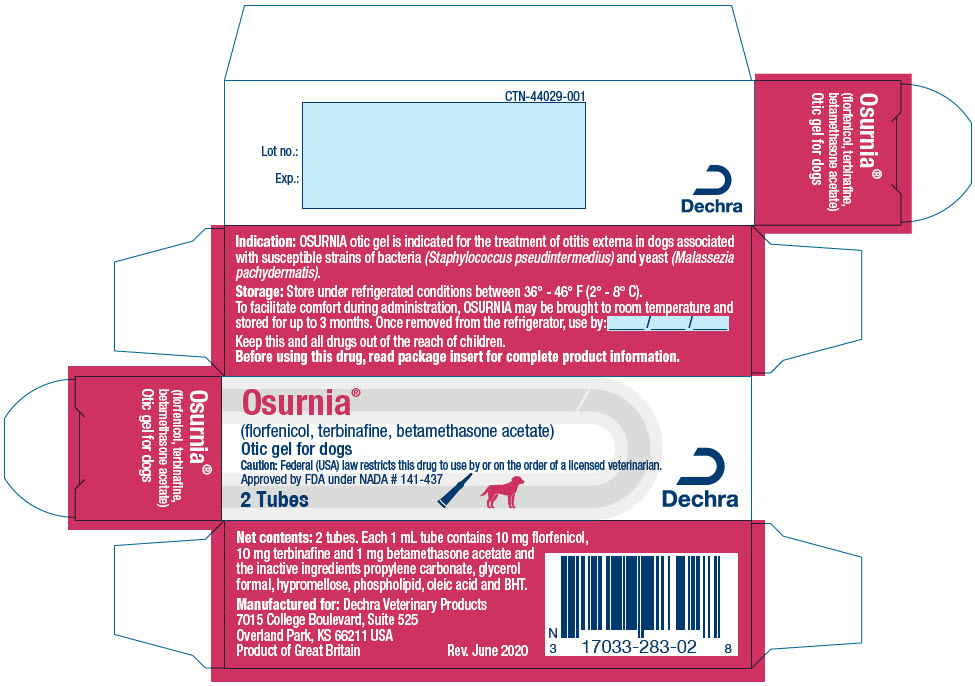
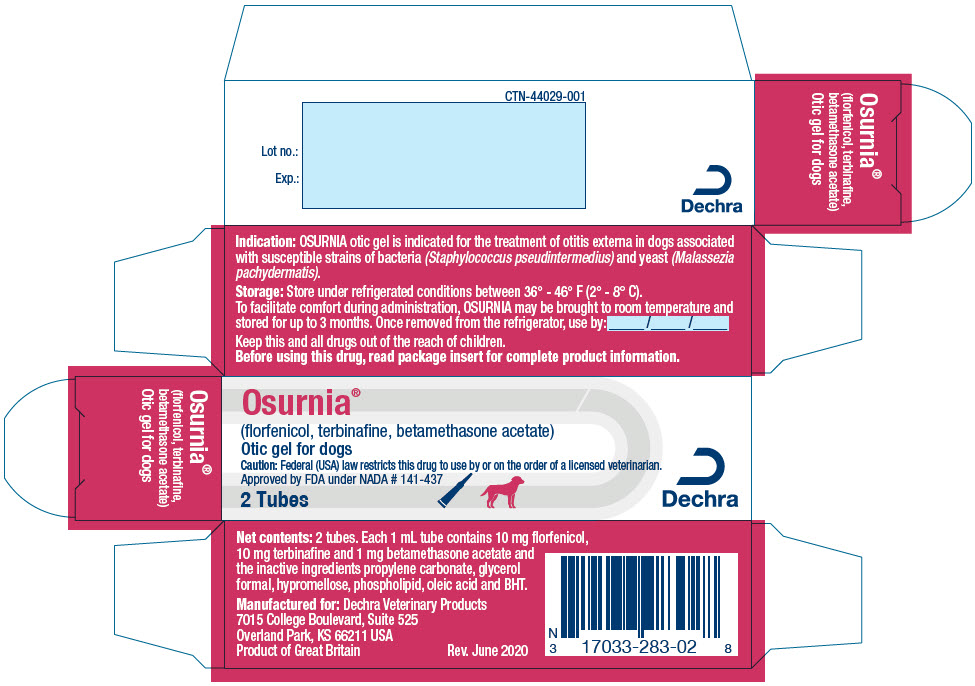
- PRINCIPAL DISPLAY PANEL - 1 Dose Tube Label
- PRINCIPAL DISPLAY PANEL - 20 Tube Carton
-
INGREDIENTS AND APPEARANCE
OSURNIA
florfenicol, terbinafine, betamethasone acetate gelProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-283 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength florfenicol (UNII: 9J97307Y1H) (florfenicol - UNII:9J97307Y1H) florfenicol 10 mg in 1 mL terbinafine (UNII: G7RIW8S0XP) (terbinafine - UNII:G7RIW8S0XP) terbinafine 10 mg in 1 mL betamethasone acetate (UNII: TI05AO53L7) (betamethasone - UNII:9842X06Q6M) betamethasone acetate 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-283-02 2 in 1 CARTON 1 1 mL in 1 TUBE, WITH APPLICATOR 2 NDC: 17033-283-20 20 in 1 CARTON 2 1 mL in 1 TUBE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141437 05/01/2015 Labeler - Dechra Veterinary Products (362142734)
Trademark Results [Osurnia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OSURNIA 77905085 4486660 Live/Registered |
ELANCO TIERGESUNDHEIT AG 2010-01-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.