Dextrose by B. Braun Medical Inc. DEXTROSE solution
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 100 mL of 70% Dextrose Injection USP contains:
Hydrous Dextrose USP 70 g; Water for Injection USP qs
pH: 4.0 (3.2-6.5); Calculated Osmolarity: 3532 mOsmol/liter, hypertonic.
Specific gravity 1.233 at 25°C
Calories per liter: 2380 calculated on the basis of 3.4 kcal/g of dextrose, hydrous
70% Dextrose Injection USP is sterile, nonpyrogenic and contains no bacteriostatic or antimicrobial agents. This product is intended for intravenous administration after appropriate admixture or dilution as a caloric source.
The formula of the active ingredient is:
Ingredient Molecular Formula Molecular Weight Hydrous Dextrose USP 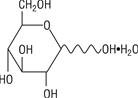
198.17 Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during use. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
-
CLINICAL PHARMACOLOGY
70% Dextrose Injection USP provides calories and is a source of water for hydration. It is capable of inducing diuresis depending on the clinical condition of the patient.
Dextrose is readily metabolized, may decrease losses of body protein and nitrogen, promotes glycogen deposition and decreases or prevents ketosis if sufficient doses are provided.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
-
INDICATIONS AND USAGE
70% Dextrose Injection USP is indicated as a caloric component in a parenteral nutrition regimen. 70% Dextrose Injection USP is used with an appropriate protein (nitrogen) source in the prevention of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns.
-
CONTRAINDICATIONS
The infusion of 70% Dextrose Injection USP is contraindicated in patients having intracranial or intraspinal hemorrhage, in patients who are severely dehydrated, in patients who are anuric, and in patients in hepatic coma.
Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
-
WARNINGS
This injection is for compounding only, not for direct infusion.
Dilute before use to a concentration which will, when administered with an amino acid (nitrogen) source, result in an appropriate calorie to gram of nitrogen ratio and which has an osmolarity consistent with the route of administration.
Unless appropriately diluted, the infusion of hypertonic dextrose injection into a peripheral vein may result in vein irritation, vein damage, and thrombosis. Strongly hypertonic nutrient solutions should only be administered through an indwelling intravenous catheter with the tip located in a large central vein such as the superior vena cava.
Use of 70% Dextrose Injection USP to prepare parenteral nutritional admixtures may be incompatible with other components, especially calcium and phosphate salts and lipid emulsions. Incompatibility of admixed components can produce precipitates which may cause particulate emboli. Use 70% Dextrose Injection USP only to prepare formulations that are known to be stable: refer to standard texts for further information.
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration.
WARNING: 70% Dextrose Injection USP contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Prolonged infusion of isotonic or hypotonic dextrose in water may increase the volume of extracellular fluid and cause water intoxication.
Solutions containing dextrose without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility of agglomeration.
Excessive administration of potassium-free dextrose solutions may result in significant hypokalemia. Serum potassium levels should be maintained and potassium supplemented as required.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
-
PRECAUTIONS
General
This solution should be used with care in patients with hypervolemia, renal insufficiency, urinary tract obstruction, or impending or frank cardiac decompensation.
Solutions containing dextrose should be used with caution in patients with overt or known subclinical diabetes mellitus or carbohydrate intolerance for any reason.
Essential electrolytes, minerals, and vitamins should be supplied as needed.
Hypokalemia may develop during parenteral administration of hypertonic dextrose solutions. Sufficient amounts of potassium should be added to dextrose solutions administered to fasting patients with good renal function, especially those on digitalis therapy.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration. See WARNINGS.
Do not use plastic container in series connection.
If administration of 70% Dextrose Injection USP after admixture or dilution is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
This solution is intended for intravenous administration after admixture or dilution using sterile equipment. When using an automated compounding device replace all disposable components as recommended by manufacturer and at least every 24 hours.
Aseptic technique is essential with the use of sterile preparations for compounding nutritional admixtures. Discard container within 4 hours of entering closure.
Administration of hypertonic dextrose and amino acid solutions via central venous catheter may be associated with complications which can be prevented or minimized by careful attention to all aspects of the procedure. This includes attention to solution preparation, administration and patient monitoring.
It is essential that a carefully prepared protocol, based upon current medical practice, be followed, preferably by an experienced team. The package insert of the protein (nitrogen) source should be consulted for dosage and all precautionary information.
Use only if solution is clear and container and seals are intact.
70% Dextrose Injection USP contains no more than 25 mcg/L of aluminum.
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require tailoring of the electrolyte pattern, in these or alternative solutions.
Drug Interactions
Caution must be exercised in the administration of 70% Dextrose Injection USP to patients receiving corticosteroids or corticotropin. Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Dispose of any unused product. See WARNINGS.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Dextrose Injections USP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
There are no adequate and well controlled studies with Dextrose Injections, USP in pregnant women and animal reproduction studies have not been conducted with this drug. Therefore, it is not known whether Dextrose Injections USP can cause fetal harm when administered to a pregnant woman. Dextrose Injections USP should be given during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
Intrapartum maternal intravenous infusion of glucose-containing solutions may produce maternal hyperglycemia with subsequent fetal hyperglycemia and fetal metabolic acidosis. Fetal hyperglycemia can result in increased fetal insulin levels which may result in neonatal hypoglycemia following delivery. Consider the potential risks and benefits for each specific patient before administering Dextrose Injection, USP.
Nursing Mothers
It is not known if this drug is present in human milk. Because many drugs are present in human milk, caution should be exercised when Dextrose Injections USP are administered to a nursing woman.
Pediatric Use
The use of Dextrose in pediatric patients is based on clinical practice (see DOSAGE AND ADMINISTRATION). Because of their hypertonicity, 70% Dextrose Injections must be diluted prior to administration.
Newborns – especially those born premature and with low birth weight - are at increased risk of developing hypo- or hyperglycemia and therefore need close monitoring during treatment with intravenous glucose solutions to ensure adequate glycemic control in order to avoid potential long term adverse effects. Hypoglycemia in the newborn can cause prolonged seizures, coma and brain damage. Hyperglycemia has been associated with intraventricular hemorrhage, late onset bacterial and fungal infection, retinopathy of prematurity, necrotizing enterocolitis, bronchopulmonary dysplasia, prolonged length of hospital stay, and death.
Geriatric Use
An evaluation of literature revealed no clinical experience identifying differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
See WARNINGS.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. Incompatibility of admixed components can produce precipitates which may cause particulate emboli.
Hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause hypovolemia, dehydration, mental confusion and/or loss of consciousness. Too rapid infusion of hypertonic solutions may cause local pain and venous irritation. Rate of administration should be adjusted according to tolerance. Use of the largest peripheral vein and a small bore needle is recommended. (See DOSAGE AND ADMINISTRATION.)
Hypersensitivity reactions, including anaphylaxis and chills.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
This solution is for intravenous use only after admixture or dilution.
70% Dextrose Injection USP is designed for use with automated compounding devices for preparing intravenous nutritional admixtures or for the filling of empty sterile syringes. Dosages will be in accordance with the recommendation of the prescribing physician. 70% Dextrose Injection USP is not intended for direct infusion. Admixtures should be made by, or under the direction of, a pharmacist using strict aseptic technique under a laminar flow hood. Compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal from refrigeration.
Dosage is to be directed by a physician and is dependent upon age, weight, clinical condition of the patient and laboratory determinations. Frequent laboratory determinations and clinical evaluation are essential to monitor changes in blood glucose and electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
Fluid administration should be based on calculated maintenance or replacement fluid requirements for each patient.
Pediatric Use
The dosage selection and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia. Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants. The infusion rate and volume depends on the age, weight, clinical and metabolic conditions of the patient, concomitant therapy and should be determined by the consulting physician experienced in pediatric intravenous fluid therapy.
-
Directions for Use of Pharmacy Bulk Package Container
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration or admixture and final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration, whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions where possible.
70% Dextrose Injection USP in the Pharmacy Bulk Package is intended for use in the preparation of sterile, intravenous admixtures.
Refer to standard texts and guidelines on the preparation of parenteral nutritional admixtures.
When compounding admixtures, use aseptic technique. Mix thoroughly.
Do not store any unused portion of 70% Dextrose Injection USP.
TO OPEN:
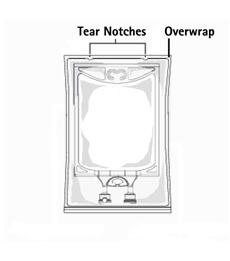
- Inspect overwrap. Do not use if overwrap has been damaged.
- Do not use unless solution is clear and closure is intact.
- Tear overwrap starting from the tear notches. (Figure 1)
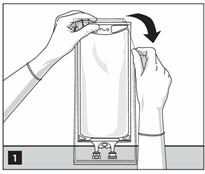
- Inspect the container for minute leaks by squeezing inner bag firmly. If leaks are found, discard the bag as sterility may be impaired.
- For compounding only. Do not use for direct infusion
PREPARATION FOR ADMIXING
Note: Important Admixing Information
- The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar air flow hood (or an equivalent clean air compounding area).
- The contents are restricted to the preparation of admixtures for infusion or, through a sterile transfer device, for the filling of empty sterile syringes.
- Additives may be incompatible with the fluid withdrawn from this container. When compounding admixtures, use aseptic technique, mix thoroughly and do not store.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution container permits. (see PRECAUTIONS, General)
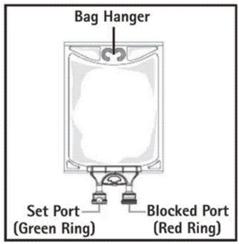
- Do not use/penetrate blocked port (see Figure 2, left upper corner).
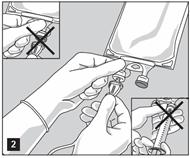
- Remove aluminum foil of set port at the bottom of container.
- Attach suitable transfer device or compounding set (Figure 2). Refer to complete directions accompanying device.
- Hang bag on suitable fixture (Figure 3).
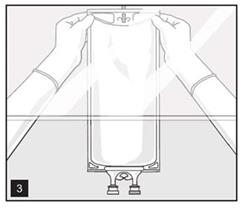
- Once container closure has been penetrated, withdrawal of content should be completed within 4 hours.
-
HOW SUPPLIED
70% Dextrose Injection USP is supplied in 2000 mL Pharmacy Bulk Package containers packaged 4 per case.
NDC REF SIZE
0264-7387-50 S8705 2000 mLExposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
70% Dextrose Injection USP
Pharmacy Bulk Package
Not For Direct Infusion
Must Be DilutedNDC: 0264-7387-50
LOT
EXP.2000 mL
Each 100 mL contains:
Hydrous Dextrose USP 70 g
Water for Injection USP qsHypertonic
Sterile. Pharmacy bulk package container.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS SHOULD
NOT BE GIVEN CONJOINTLY WITH BLOOD BECAUSE AGGLOMERATION
MAY OCCUR. Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.CONTAINS NO MORE THAN 25 mcg/L OF ALUMINUM
Recommended Storage: Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert. Do not remove
overwrap until ready for use.Not made with natural rubber latex, PVC or DEHP.
REF S8705
Rx only

Y38-000-052 LD-251-6
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
SET
-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7387 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 70 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7387-50 4 in 1 CASE 02/19/2015 1 2000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019626 02/19/2015 Labeler - B. Braun Medical Inc. (002397347) Establishment Name Address ID/FEI Business Operations B. Braun Medical Inc. 037425308 LABEL(0264-7387) , MANUFACTURE(0264-7387) , PACK(0264-7387)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.