Get-Back - Targeted Body Acne Spray
Get-Back - Targeted Body Acne by
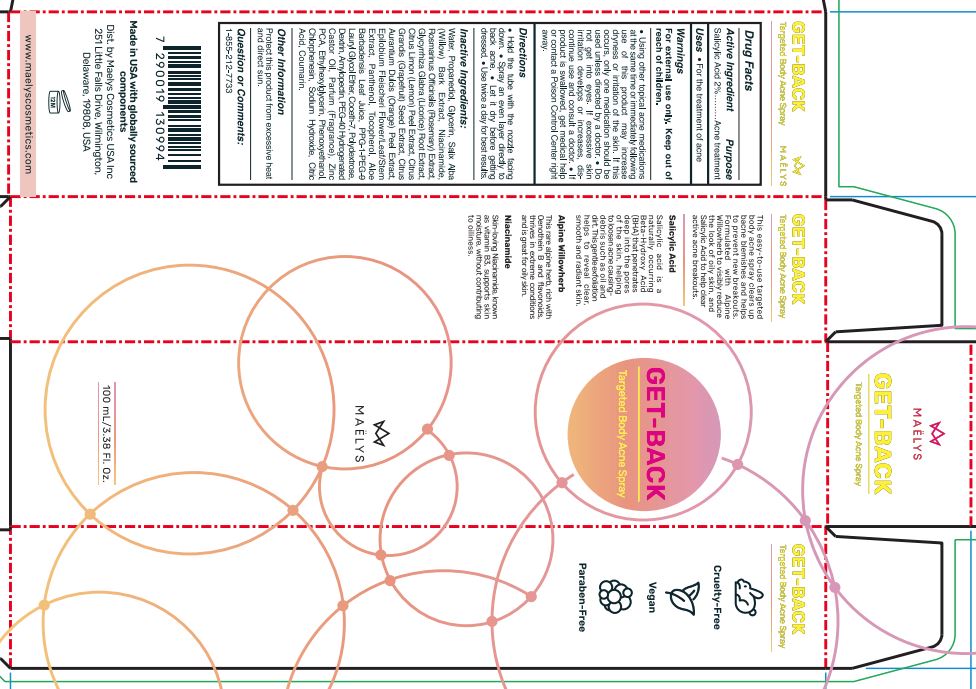
Drug Labeling and Warnings
Get-Back - Targeted Body Acne by is a Otc medication manufactured, distributed, or labeled by Maelys Cosmetics, LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GET-BACK - TARGETED BODY ACNE- salicylic acid spray
Maelys Cosmetics, LTD
----------
Get-Back - Targeted Body Acne Spray
Warnings
For external use only.
Keep out of reach of children.
Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor. Do not get into eyes. tt excessive skin irritation develops or increases, discontinue use and consult a doctor. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
Hold the tube with the nozzle facing down. Spray an even layer directly to back acne. Let it dry before getting dressed. Use twice a day for best results.
Inactive Ingredients:
Water, Propanediol, Glycerin, Salix Alba (Willow) Bark Extract, Niacinamide, Rosmarinus Officinalis (Rosemary) Extract, Glycyrrhiza Glabra (Licorice) Root Extract. Citrus Limon (Lemon) Peel Extract, Citrus Grandis (Grapefruit) Seed Extract, Citrus Aurantium Dulcis (Orange) Peel Extract, Epilobium Fleischeri Flower/Leaf/Stem Extract, Panthenol, Tocopherol, Aloe Barbadensis Leaf Juice, PPG-1-PEG-9 Lauryl Glycol Ether, Coceth-7, Polydextrose, Dextrin, Amylopectin, PEG-40 Hydrogenated Castor Oil, Parfum (Fragrance), Zinc PCA, Ethylhexylglycerin, Phenoxyethanol, Chlorphenesin, Sodium Hydroxide, Citric Acid, Coumarin.
| GET-BACK - TARGETED BODY ACNE
salicylic acid spray |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Maelys Cosmetics, LTD (532018258) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.