Pulmosal by PharmaCaribe PULMOSAL-
Pulmosal by
Drug Labeling and Warnings
Pulmosal by is a Other medication manufactured, distributed, or labeled by PharmaCaribe. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
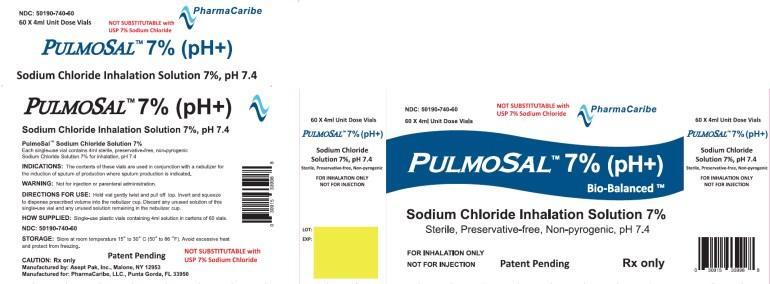
Pulmosal 7% (pH+) 4 mL
Sodium Chloride Inhalation Solution 7%,
Sterile, Preservative-free, Non-pyrogenic, pH 7.4Each Single-use vial contains 4ml sterile, preservative-free, non-pyrogenic Sodium Chloride Solution 7% for inhalation, pH 7.4
FOR INHALATION ONLYNOT FOR INJECTION.
Patent Pending
RX Only60 x 4 mL Unit-Dose Vial
NOT SUBSTITUTABLE with USP 7% Sodium Chloride
- INDICATIONS AND USAGE
- INSTRUCTIONS FOR USE SECTION
- How Supplied
- WARNINGS
- STORAGE
- Pulmosal 7% (pH+) 4 mL
-
INGREDIENTS AND APPEARANCE
PULMOSAL
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:50190-740 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698, SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 280 mg in 4 mL Product Characteristics number of times usable 1 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50190-740-60 60 in 1 BOX 1 4 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device CAF 08/17/2010 Labeler - PharmaCaribe (011702541) Registrant - PharmaCaribe (011702541) Establishment Name Address ID/FEI Business Operations PharmaCaribe 011702541 label
Trademark Results [Pulmosal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PULMOSAL 85682088 4308546 Live/Registered |
Pharmacaribe 2012-07-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.