Dr. Evans Oil-Free DEFENSE DAY TINTED MOISTURIZER SPF 55 by Guthy-Renker, LLC. / AMCOL Health & Beauty Solutions, Incorporated / Thibiant, International, Inc.
Dr. Evans Oil-Free DEFENSE DAY TINTED MOISTURIZER SPF 55 by
Drug Labeling and Warnings
Dr. Evans Oil-Free DEFENSE DAY TINTED MOISTURIZER SPF 55 by is a Otc medication manufactured, distributed, or labeled by Guthy-Renker, LLC., AMCOL Health & Beauty Solutions, Incorporated, Thibiant, International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
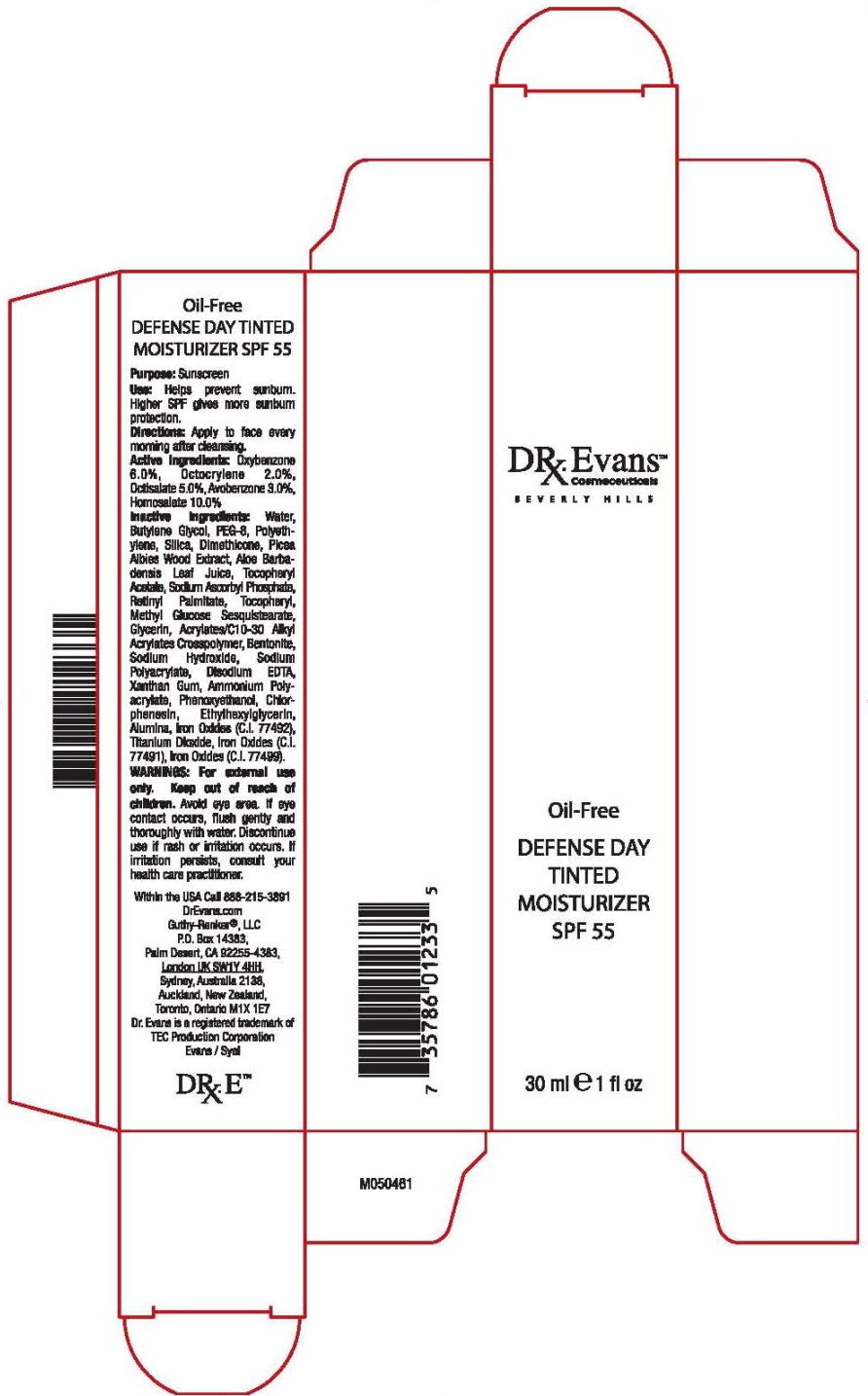
DR. EVANS OIL-FREE DEFENSE DAY TINTED MOISTURIZER SPF 55- homosalate, octisalate, oxybenzone, avobenzone, octocrylene liquid
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients:
Oxybenzone 6.0%, Octocrylene 2.0%, Octisalate 5.0%, Avobenzone 3.0%, Homosalate 10.0%
Inactive Ingredients:
Water, Butylene Glycol, PEG-8, Polyethylene, Silica, Dimethicone, Picea Albies Wood Extract, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Sodium Ascorbyl Phosphate, Retinyl Palmitate, Tocopheryl, Methyl Glucose Sesquistearate, Glycerin, Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Bentonite, Sodium Hydroxide, Sodium Polyacrylate, Disodium EDTA, Xanthan Gum, Ammonium Polyacrylate, Phenoxyethanol, Chlorphenesin, Ethylhexylglycerin, Alumina, Iron Oxides (C.I. 77492), Titanium Dioxide, Iron Oxides (C.I. 77491, Iron Oxides (C.I. 77499).
WARNINGS:
For external use only. Keep out of reach of children. Avoid eye area, if eye contact occurs, flush gently and thoroughly with water. Discontinue use if rash or irritation occurs. If irritation persists, consult your health care practitioner.
Within the USA Call 888-215-3891
DrEvans.com
Guthy-Renker®, LLC
P.O. Box 14383,
Palm Desert, CA 92555-4383,
London UK SW1Y 4HH,
Sydney, Australia 2138,
Auckland, New Zealand,
Toronto, Ontario M1X 1E7
Dr. Evans is a registered trademark of
TEC Production Corporation
Evans/Syal
DR. E tm
| DR. EVANS OIL-FREE DEFENSE DAY TINTED MOISTURIZER SPF 55
homosalate, octisalate, oxybenzone, avobenzone, octocrylene liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Registrant - AMCOL Health & Beauty Solutions, Incorporated (872684803) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMCOL Health & Beauty Solutions, Incorporated | 872684803 | manufacture(11410-411) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Thibiant, International, Inc. | 083913913 | manufacture(11410-411) | |