DESONATE by Bayer HealthCare Pharmaceuticals Inc. / Contract Pharmaceutical Limited DESONATE- desonide gel
DESONATE by
Drug Labeling and Warnings
DESONATE by is a Prescription medication manufactured, distributed, or labeled by Bayer HealthCare Pharmaceuticals Inc., Contract Pharmaceutical Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DESONATE® Gel safely and effectively. See full prescribing information for DESONATE Gel.
DESONATE (desonide) Gel 0.05% for topical use only
Initial U.S. Approval: 1972INDICATIONS AND USAGE
Desonate® is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older. (1)
DOSAGE AND ADMINISTRATION
- Apply as a thin layer to the affected areas two times daily and rub in gently. (2)
- Therapy should be discontinued when control is achieved. (2)
- If no improvement is seen within 4 weeks, reassessment of diagnosis may be necessary. (2)
- Should not be used with occlusive dressings. (2)
- Treatment beyond 4 consecutive weeks is not recommended. (2)
For topical use only. Not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Gel, 0.05%; (0.5mg/g) desonide in a translucent to opaque gel (3)
CONTRAINDICATIONS
History of hypersensitivity to any of the components of the preparation. (4)
WARNINGS AND PRECAUTIONS
- Topical corticosteroids can produce reversible hypothalamic pituitary adrenal (HPA) axis suppression, Cushing's syndrome and unmask latent diabetes. (5.1)
- Systemic absorption may require evaluation for HPA axis suppression (5.1).
- Modify use should HPA axis suppression develop (5.1)
- Potent corticosteroids, use on large areas, prolonged use or occlusive use may increase systemic absorption (5.1)
- Local adverse reactions may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis and may be more likely with occlusive use or more potent corticosteroids. (5.2, 5.4, 6)
- Children may be more susceptible to systemic toxicity when treated with topical corticosteroids. (5.1, 8.4)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 1%) are headache and application site burning. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Safety and effectiveness of Desonate in pediatric patients less than 3 months of age have not been evaluated, and therefore its use in this age group is not recommended. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
5.2 Local Adverse Reactions with Topical Corticosteroids
5.3 Concomitant Skin Infections
5.4 Skin Irritation
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Desonate® is indicated for the treatment of mild to moderate atopic dermatitis in patients 3 months of age and older.
Patients should be instructed to use Desonate for the minimum amount of time as necessary to achieve the desired results because of the potential for Desonate to suppress the hypothalamic-pituitary-adrenal (HPA) axis [see Warnings and Precautions (5.1)]. Treatment should not exceed 4 consecutive weeks [see Dosage and Administration (2)].
-
2 DOSAGE AND ADMINISTRATION
Apply a thin layer to the affected areas two times daily and rub in gently. Discontinue use when control is achieved. If no improvement is seen within 4 weeks, reassessment of diagnosis may be necessary. Treatment beyond 4 consecutive weeks is not recommended. Do not use with occlusive dressings. Avoid contact with eyes or other mucous membranes.
For topical use only. Not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of the topical corticosteroid.
The effect of Desonate on HPA axis function was investigated in pediatric subjects, 6 months to 6 years old, with atopic dermatitis covering at least 35% of their body, who were treated with Desonate twice daily for 4 weeks. One of 37 subjects (3%) displayed adrenal suppression after 4 weeks of use, based on the cosyntropin stimulation test. As follow-up evaluation of the subject's adrenal axis was not performed, it is unknown whether the suppression was reversible [see Use In Specific Populations (8.4) and Clinical Pharmacology (12.2)].
Pediatric patients may be more susceptible than adults to systemic toxicity from equivalent doses of Desonate due to their larger skin surface-to-body mass ratios [see Use In Specific Populations (8.4)].
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas, use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure.
An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure.
5.2 Local Adverse Reactions with Topical Corticosteroids
Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include skin atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible.
5.3 Concomitant Skin Infections
If concomitant skin infections are present or develop during treatment, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of Desonate should be discontinued until the infection is adequately controlled.
5.4 Skin Irritation
If irritation develops, Desonate should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical studies of 425 Desonate treated subjects and 157 Vehicle-treated subjects, adverse events occurred at the application site in 3% of subjects treated with Desonate and the incidence rate was not higher compared with vehicle-treated subjects. The most common local adverse events in Desonate treated subjects were application site burning in 1% (4/425) and rash in 1% (3/425) followed by application site pruritus in <1% (2/425).
Adverse events that resulted in premature discontinuation of study drug in Desonate treated subjects were telangiectasia and worsening of atopic dermatitis in one subject each. Additional adverse events observed during clinical trials for patients treated with Desonate included headache in 2% (8/425) compared with 1% (2/157) in those treated with vehicle.
The following additional local adverse reactions have been reported infrequently with topical corticosteroids. They may occur more frequently with the use of occlusive dressings, especially with higher potency corticosteroids. These reactions are listed in an approximate decreasing order of occurrence: folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, secondary infection, skin atrophy, striae, and miliaria.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category C:
There are no adequate and well-controlled studies in pregnant women. Therefore, Desonate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
No reproductive studies in animals have been performed with Desonate. Dermal embryofetal development studies were conducted in rats and rabbits with a desonide cream, 0.05% formulation. Topical doses of 0.2, 0.6, and 2.0 g cream/kg/day of a desonide cream, 0.05% formulation or 2.0 g/kg of the cream base were administered topically to pregnant rats (gestational days 6-15) and pregnant rabbits (gestational days 6-18). Maternal body weight loss was noted at all dose levels of the desonide cream, 0.05% formulation in rats and rabbits. Teratogenic effects characteristic of corticosteroids were noted in both species. The desonide cream, 0.05% formulation was teratogenic in rats at topical doses of 0.6 and 2.0 g cream/kg/day and in rabbits at a topical dose of 2.0 g cream/kg/day. No teratogenic effects were noted for the desonide cream, 0.05% formulation at a topical dose of 0.2 g cream/kg/day in rats and 0.6 g cream/kg/day in rabbits. These doses (0.2 g cream/kg/day and 0.6 g cream/kg/day) are similar to the maximum recommended human dose based on body surface area comparisons.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Desonate is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of Desonate in pediatric patients less than 3 months of age have not been evaluated, and therefore its use in this age group is not recommended.
The effect of Desonate on HPA axis function was investigated in pediatric subjects, with atopic dermatitis covering at least 35% of their body, who were treated with Desonate twice daily for 4 weeks. One of 37 subjects (3%) displayed adrenal suppression after 4 weeks of use, based on the cosyntropin stimulation test [see Warnings and Precautions (5.1)].
In controlled clinical studies in subjects 3 months to 18 years of age, 425 subjects were treated with Desonate and 157 subjects were treated with vehicle [see Adverse Reactions (6) and Clinical Studies (14)].
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids. They are therefore also at greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing's syndrome while on treatment.
Adverse effects, including striae, have been reported with inappropriate use of topical corticosteroids in infants and children. HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
8.5 Geriatric Use
Clinical studies of Desonate did not include patients aged 65 and older to determine if they respond differently than younger patients. Treatment of this patient population should reflect the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Topically applied Desonate can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)].
-
11 DESCRIPTION
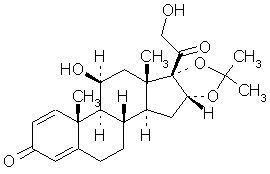
Desonate contains desonide [(pregna-1, 4-diene-3, 20-dione,11, 21-dihydroxy-16, 17-[(1-methylethylidene) bis(oxy)]-,(11β,16α)]- a synthetic nonfluorinated corticosteroid for topical dermatologic use. Chemically, desonide is C24H32O6. It has the following structural formula:
Desonide has the molecular weight of 416.52. It is a white to off-white odorless powder which is soluble in methanol and practically insoluble in water. Each gram of Desonate contains 0.5 mg of desonide in an aqueous gel base of purified water, glycerin, propylene glycol, edetate disodium dihydrate, methylparaben, propylparaben, sodium hydroxide, and Carbopol® 981.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
In an HPA axis suppression study, one of 37 (3%) pediatric subjects, 6 months to 6 years old, with moderate to severe atopic dermatitis covering at least 35% body surface area who applied Desonate experienced suppression of the adrenal glands following 4 weeks of therapy [see Warnings And Precautions (5.1) and Use In Specific Populations (8.4)]. A follow-up evaluation of the subject's adrenal axis was not performed; it is unknown whether the suppression was reversible.
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including product formulation and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. They are metabolized primarily in the liver and then are excreted by the kidneys. Some corticosteroids and their metabolites are also excreted in the bile.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Systemic long-term animal studies have not been performed to evaluate the carcinogenic potential of desonide or its effect on fertility. In a 26-week dermal carcinogenicity study conducted in transgenic (Tg.AC) mice, once daily application of 0.005% to 0.05% Desonate Gel significantly increased the incidence of papillomas at the treatment site in males and females compared to their respective control animals. The clinical relevance of these findings in animals to humans is not clear.
Desonide revealed no evidence of mutagenic potential based on the results of an in vitro genotoxicity test (Ames assay) and an in vivo genotoxicity test (mouse micronucleus assay). Desonide was positive without S9 activation and was equivocal with S9 activation in an in vitro mammalian cell mutagenesis assay (L5178YITK+ mouse lymphoma assay). A dose response trend was not noted in this assay.
-
14 CLINICAL STUDIES
In two randomized vehicle-controlled clinical studies, subjects 3 months to 18 years of age with mild to moderate atopic dermatitis were treated twice daily for 4 weeks with either Desonate or vehicle. Treatment success was defined as achieving clear or almost clear on the Investigator's Global Severity Score (IGSS) with at least a 2-point change (decrease) from the subject's baseline IGSS when compared to the Week 4 IGSS. The results of the 2 clinical trials are summarized in Table 1:
Table 1: Subjects Achieving Treatment Success Clinical Trial 1
Desonate
N = 289
Vehicle
N = 92
128 (44%)
13 (14%)
Clinical Trial 2
Desonate
N = 136
Vehicle
N = 65
38 (28%)
4 (6%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Desonate is a translucent to opaque gel supplied in 60g tubes in cartons containing 1x 60g tube (NDC: 50419-828-06), or a 2 x 60g tube TwinPack (NDC: 50419-828-12). .
Storage:
Store at 25°C (77°F); excursions permitted to15-30°C (59-86°F). [See USP Controlled Room Temperature].
Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- This medication should not be used for any disorder other than that for which it was prescribed.
- Unless directed by the physician, the treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive.
- Unless directed by a physician, this medication should not be used on the underarm or groin areas of pediatric patients.
- Parents of pediatric patients should be advised not to use Desonate in the treatment of diaper dermatitis. Desonate should not be applied in the diaper area, as diapers or plastic pants may constitute occlusive dressing [see Dosage and Administration (2)].
- Patients should report to their physician any signs of local adverse reactions.
- Other corticosteroid-containing products should not be used with Desonate without first consulting with the physician.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 4 weeks, contact the physician.
© 2014, Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981
Manufactured in Canada
Rev. July 2014
6706906
The following is a representative example of Desonate labeling. See the "How Supplied" section for a complete listing of all components.
-
PRINCIPAL DISPLAY PANEL
Desonate 60 g Carton
NDC: 50419-828-06
Desonate®
(desonide) Gel 0.05%
Rx Only Net Wt 60 g
For Topical Use Only. Not for Ophthalmic, Oral, or Intravaginal Use.
-
INGREDIENTS AND APPEARANCE
DESONATE
desonide gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-828 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESONIDE (UNII: J280872D1O) (DESONIDE - UNII:J280872D1O) DESONIDE 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-828-06 1 in 1 CARTON 07/23/2019 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 50419-828-12 2 in 1 CARTON 07/23/2019 2 60 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 50419-828-72 1 in 1 CARTON 07/23/2019 3 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021844 10/20/2006 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Contract Pharmaceutical Limited 248761249 MANUFACTURE(50419-828)
Trademark Results [DESONATE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DESONATE 78356061 3276136 Live/Registered |
LEO PHARMA A/S 2004-01-22 |
 DESONATE 77226193 3531883 Live/Registered |
LEO PHARMA A/S 2007-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.