Dr.Graft by Medicosbiotech. Inc / PL COSMETIC
Dr.Graft by
Drug Labeling and Warnings
Dr.Graft by is a Otc medication manufactured, distributed, or labeled by Medicosbiotech. Inc, PL COSMETIC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
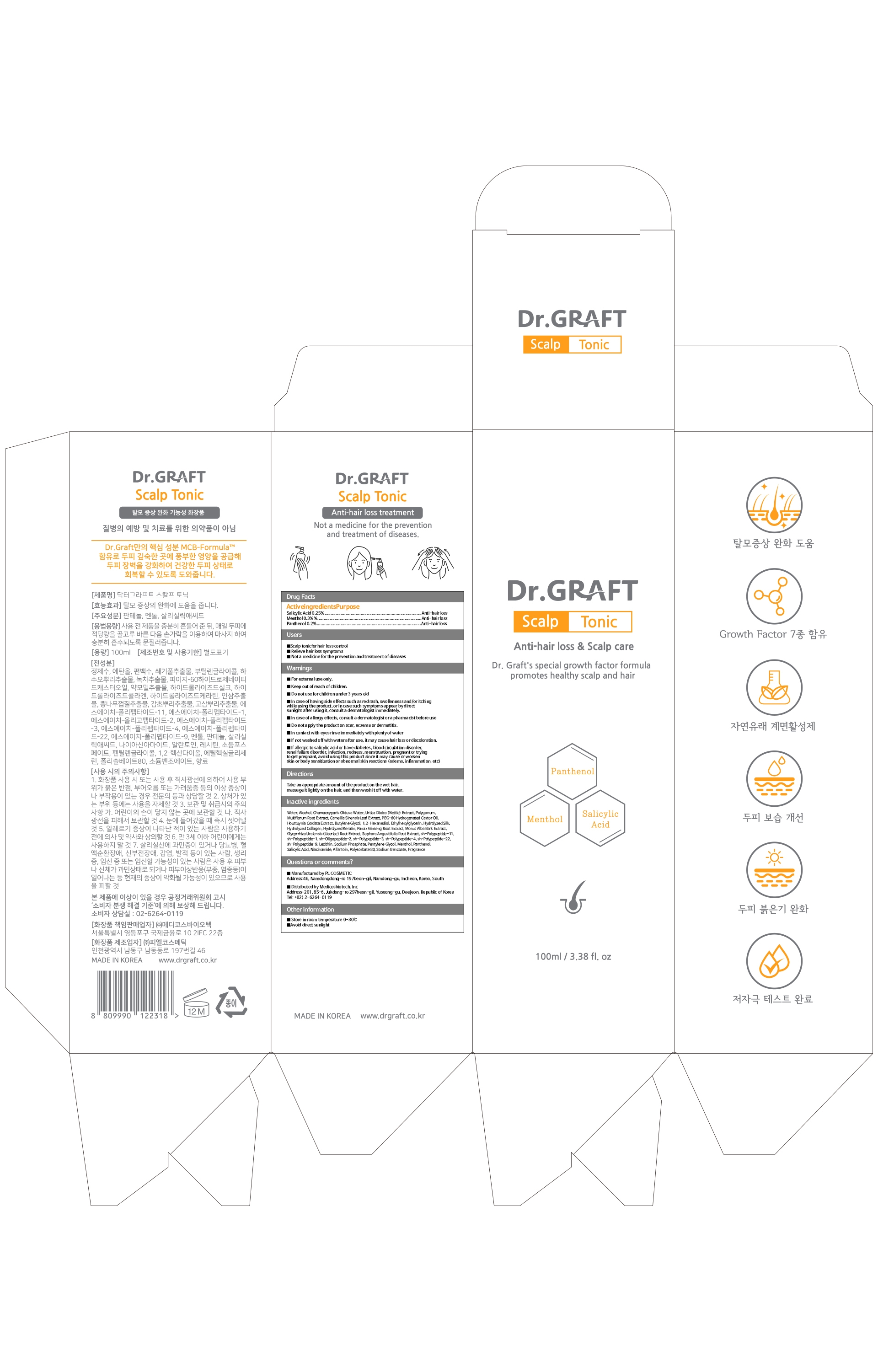
DR.GRAFT- scalp tonic liquid
Medicosbiotech. Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Use
Scalp tonic for hair loss control
Relieve hair loss symptoms
Not a medicine for the prevention and treatment of diseases
Warnings
For external use only.
Keep out of reach of children.
Do not use for children under 3 years old.
In case of having side effects such as red rash, swollenness and/or itching while using the product, or in case such symptoms appear by direct sunlight after using it, consult a dermatologist immediately.
Keep out of reach of children.
In case of allergy effects, consult a dermatologist or a pharmacist before use
Do not apply the product on scar, eczema or dermatitis.
In contact with eyes rinse immediately with plenty of water
If not washed off with water after use, it may cause hair loss or discoloration.
If allergic to salicylic acid or have diabetes, blood circulation disorder, renal failure disorder, infection, redness, menstruation, pregnant or trying
to get pregnant, avoid using this product since it may cause or worsen skin or body sensitization or abnormal skin reactions (edema, inflammation, etc)
Directions
Take an appropriate amount of the product on the wet hair, massage it lightly on the hair, and then wash it off with water.
Inactive ingredients
Water, Disodium Laureth Sulfosuccinate, Cocamidopropyl Betaine, Urtica Dioica (Nettle) Extract,
Cocamide MIPA, PEG-7 Glyceryl Cocoate, Salicylic Acid, Houttuynia Cordata Extract, Butylene Glycol,
1,2-Hexanediol, Ethylhexylglycerin, Hydrolyzed Silk, Hydrolyzed Collagen, Hydrolyzed Keratin,
Copper Tripeptide-1, Panax Ginseng Root Extract, Morus Alba Bark Extract,
Cnidium Officinale Root Extract, Acorus Calamus Root Extract, sh-Polypeptide-11, sh-Polypeptide-1,
sh-Oligo peptide-2, sh-Polypeptide-3, sh-Polypeptide-4, sh-Polypep tide-22, sh-Polypeptide-9,
Lecithin, Sodium Phosphate, Pentylene Glycol, Panthenol, Menthol, Tocopheryl Acetate,
Polyquaternium-10, PEG-150 Distearate, Caprylyl Glycol, Disodium EDTA, Citric Acid, Fragrance
| DR.GRAFT
scalp tonic liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Medicosbiotech. Inc (695067951) |
| Registrant - Medicosbiotech. Inc (695067951) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.