ZUSDURI by UroGen Pharma, Inc / UroGen Pharma, Ltd. ZUSDURI- mitomycin kit
ZUSDURI by
Drug Labeling and Warnings
ZUSDURI by is a Prescription medication manufactured, distributed, or labeled by UroGen Pharma, Inc, UroGen Pharma, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZUSDURI safely and effectively. See full prescribing information for ZUSDURI.
ZUSDURI™ (mitomycin) for intravesical solution
Initial U.S. Approval: 1974INDICATIONS AND USAGE
ZUSDURI is an alkylating drug indicated for the treatment of adult patients with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC). (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risks in Patients with Perforated Bladder: Evaluate the bladder before the intravesical instillation of ZUSDURI. Do not administer to patients with a perforated bladder or in whom the integrity of the bladder mucosa has been compromised. (4, 5.1).
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and to use effective contraception. (5.2, 8.1, 8.3)
ADVERSE REACTIONS
The most common (≥ 10%) adverse reactions, including laboratory abnormalities, that occurred in patients were increased creatinine, increased potassium, dysuria, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, increased eosinophils, decreased lymphocytes, urinary tract infection, decreased neutrophils, and hematuria. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UroGen Pharma at 1-855-987-6436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dose
2.3 Preparation Instructions
2.4 Bladder Instillation of ZUSDURI
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks in Patients with Perforated Bladder
5.2 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer ZUSDURI by intravesical instillation only. Do not administer by pyelocalyceal instillation or by any other route.
2.2 Recommended Dose
The recommended dose of ZUSDURI is 75 mg (56 mL) instilled once weekly for six weeks into the bladder via a urinary catheter. [see Dosage and Administration (2.4)]
2.3 Preparation Instructions
See the Instructions for Pharmacy enclosed in the carton for complete information on preparation.
ZUSDURI must be reconstituted with sterile hydrogel under chilled conditions. Reconstituted ZUSDURI has reverse thermal properties with a gelation point of approximately 19°C (66°F) and will appear as a viscous liquid under chilled conditions and a semisolid gel at room temperature.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
ZUSDURI is a hazardous drug. Follow applicable special handling and disposal procedures.1
2.4 Bladder Instillation of ZUSDURI
See the Instructions for Administration enclosed in the carton for complete information on bladder instillation.
ZUSDURI must be chilled at -3°C to 5°C (27°F to 41°F) to convert to a viscous liquid prior to instillation. When instilling ZUSDURI, each syringe must be emptied within thirty (30) seconds to avoid gelation.
Instillation of ZUSDURI requires syringes and a urinary catheter with fixed Luer Lock connectors.
Advise patients that ZUSDURI may discolor urine to a violet to blue color following the instillation procedure. Advise patients for at least 24 hours post-instillation to avoid urine contact with skin, to void urine sitting on a toilet, to wash hands and genital area with water and soap after each urination, and to flush the toilet several times after use.
-
3 DOSAGE FORMS AND STRENGTHS
For intravesical solution: A kit containing the following:
- Two 40 mg (each) single-dose vials of sterile, lyophilized, grey to greyish-purple, cake or powder of mitomycin for intravesical solution.
- One single-dose vial of 60 mL of sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution.
-
4 CONTRAINDICATIONS
ZUSDURI is contraindicated in patients with:
- Perforation of the bladder [see Warnings and Precautions (5.1)],
- Prior hypersensitivity reactions to mitomycin or any component of the product.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks in Patients with Perforated Bladder
ZUSDURI may lead to systemic exposure to mitomycin and severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised.
Evaluate the bladder before the intravesical instillation of ZUSDURI and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored [see Contraindications (4)].
5.2 Embryo-Fetal Toxicity
Based on findings in animals and mechanism of action, ZUSDURI can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of mitomycin resulted in teratogenicity. Advise females of reproductive potential to use effective contraception during treatment with ZUSDURI and for 6 months following the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZUSDURI and for 3 months following the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
The safety of ZUSDURI was evaluated in ENVISION, a single-arm, multicenter study in 240 patients with recurrent LG-IR-NMIBC [see Clinical Studies (14)]. Patients received 75 mg ZUSDURI instilled once a week for 6 consecutive weeks. The median number of doses of ZUSDURI administered to patients was 6 (range 1-6) doses and 228 patients (95%) received all six scheduled doses.
Serious adverse reactions occurred in 12% of patients who received ZUSDURI, including urinary retention (0.8%) and urethral stenosis (0.4%). A fatal adverse reaction of cardiac failure occurred in 1 patient (0.4%) receiving ZUSDURI.
Permanent discontinuation of ZUSDURI due to an adverse reaction occurred in 2.9% of patients, including 1.7% who discontinued due to a renal or urinary disorder.
Dosage interruption of ZUSDURI due to adverse reactions occurred in 10% of patients. Adverse reactions (≥ 2%) which required dosage interruption were urinary tract infection (2.5%) and dysuria (2.5%).
The most common (≥ 10%) adverse reactions, including laboratory abnormalities, that occurred in patients were increased creatinine, increased potassium, dysuria, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, increased eosinophils, decreased lymphocytes, urinary tract infection, decreased neutrophils, and hematuria.
Table 1 summarizes the adverse reactions in ENVISION.
Table 1: Adverse Reactions (≥ 10% All Grades*) in Patients Who Received ZUSDURI in ENVISION Adverse Reaction ZUSDURI
N = 240All Grades
(%)Grade 3 or 4†
(%)- * Graded per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
- † Only includes Grade 3 adverse reactions
- ‡ Includes multiple related terms
Renal and urinary disorders Dysuria 23 0.4 Hematuria‡ 10 0 Infections and infestations Urinary tract infection‡ 12 0.8 Clinically relevant adverse reactions occurring in < 10% of patients receiving ZUSDURI in ENVISION included increased urinary frequency, fatigue, urinary incontinence, urinary retention, urethral stenosis, genital pain, urinary urgency, genital edema, genital pruritus, genital rash, urethritis, acute kidney injury, balanoposthitis, and nocturia.
Table 2 summarizes laboratory abnormalities in ENVISION.
Table 2: Laboratory Abnormalities (≥ 10% All Grades*) That Worsened From Baseline in Patients Who Received ZUSDURI in ENVISION Laboratory Abnormality ZUSDURI† All Grades
(%)Grade 3 or 4‡
(%)- * Graded per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
- † The denominator used to calculate the rate varied from 227 to 238 based on the number of patients with a baseline value and at least one post-treatment value
- ‡ Only includes Grade 3 laboratory abnormalities
Hematology Hemoglobin Decreased 17 0.8 Eosinophils Increased 15 0 Lymphocytes Decreased 14 0.4 Neutrophils Decreased 10 0.4 Chemistry Creatinine Increased 29 1.3 Potassium Increased 26 2.2 Aspartate Aminotransferase (AST) Increased 15 0.4 Alanine Aminotransferase (ALT) Increased 15 0.4 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and mechanism of action, ZUSDURI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on ZUSDURI use in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of mitomycin resulted in teratogenicity (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mitomycin in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ZUSDURI and for 1 week following the last dose.
8.3 Females and Males of Reproductive Potential
ZUSDURI can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating ZUSDURI.
8.5 Geriatric Use
Of the 240 patients receiving ZUSDURI in the ENVISION study, 162 (68%) were 65 years of age and older and 89 (37%) were 75 years of age and older. No significant overall differences in safety or efficacy were observed in patients 65 years of age and older, and in patients 75 years of age and older, compared to younger patients.
8.6 Renal Impairment
Avoid use of ZUSDURI in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] < 30 mL/min). A higher incidence of hematuria and urinary tract infections was observed in patients with moderate renal impairment (eGFR 30 to <60 mL/min). Monitor patients with moderate renal impairment for increased adverse reactions. No dosage adjustments are recommended in patients with mild (eGFR 60 to <90 mL/min) or moderate renal impairment.
-
11 DESCRIPTION
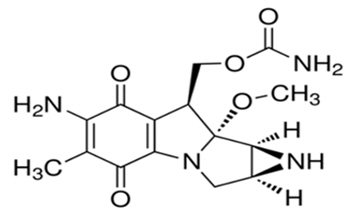
Mitomycin (also known as mitomycin-C) is an alkylating drug isolated from the broth of Streptomyces. Mitomycin is a blue-violet crystalline powder with a molecular formula of C15H18N4O5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane, and it has the following structural formula:
Mitomycin is heat stable, has a high melting point, and is freely soluble in organic solvents.
ZUSDURI is supplied in a kit containing two vials of sterile lyophilized mitomycin for intravesical solution, 40 mg each, and one vial of 60 mL of sterile hydrogel, to be used as a vehicle for reconstitution.
Mitomycin for intravesical solution is a sterile, lyophilized, grey to greyish-purple, cake or powder that contains mitomycin 40 mg and mannitol 80 mg in each vial.
Hydrogel is a sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), which contains 0.11 g hydroxypropyl methylcellulose, 17.12 g poloxamer, 0.63 g polyethylene glycol, and water for injection in each vial.
Once reconstituted, ZUSDURI is a clear, purple, viscous liquid at 2°C to 8°C (36°F to 46°F) or semisolid gel at room temperature, which may contain a few visible particles and have a pH between 6.0 and 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mitomycin inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
12.2 Pharmacodynamics
Mitomycin exposure-response relationships and time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The systemic exposure of mitomycin following instillation of 75 mg of mitomycin as ZUSDURI into the bladder was evaluated pre-instillation and hourly for up to six hours post-instillation in six patients. Mitomycin mean (range) maximum concentration (Cmax) is 2.3 ng/mL (0.2 to 8.9 ng/mL), which is less than 1% of the expected Cmax after intravenous administration.
Metabolism
Mitomycin is metabolized primarily in the liver, but metabolism occurs in other tissues as well. It is believed that the rate of clearance is inversely proportional to the maximal serum concentration because of saturation of the degradative pathways.
Excretion
Following instillation into the bladder, ZUSDURI forms a semisolid gel which dissolves in the urine. Patients reported visible gel in urine for up to 24 hours (median 5 hours) after instillation. Mitomycin is excreted unchanged in the urine. Systemically absorbed mitomycin is rapidly cleared from the serum and approximately 10% is excreted unchanged in the urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to evaluate carcinogenic potential from instillation of mitomycin into the bladder have not been conducted. Mitomycin has been found to be carcinogenic in rats and mice. At doses approximating the recommended intravenous clinical dose in humans, mitomycin produced a greater than 100% increase in tumor incidence in male Sprague-Dawley rats, and a greater than 50% increase in tumor incidence in female Swiss mice.
The effect of ZUSDURI on fertility is unknown.
-
14 CLINICAL STUDIES
ENVISION Study
The efficacy of ZUSDURI was evaluated in ENVISION (NCT05243550), a single-arm, multicenter trial in 240 adults with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC), of whom 223 were evaluable for response.
LG-IR-NMIBC was defined as Ta disease, histologically confirmed by biopsy, having one or two of the following: the presence of multiple tumors, a solitary tumor > 3 cm, and/or early or frequent recurrence (≥ 1 occurrence of LG-NMIBC within 1 year of the current diagnosis). Patients were required to have a previous occurrence of LG-NMIBC (Ta) treated by TURBT. The trial excluded patients with T1 tumors, or history of high-grade NMIBC within the previous two years, and/or those with prior intravesical chemotherapy within the prior two years (except for a single dose of intravesical chemotherapy immediately after any previous TURBT) and/or Bacillus Calmette-Guerin treatment within the previous year.
Patients received 75 mg ZUSDURI via urinary catheter once a week for 6 weeks.
Assessment of tumor status was performed every 3 months by cystoscopy, for-cause biopsy, and urine cytology. The major efficacy outcome measures were complete response rate (CR) at 3 months (defined as no detectable disease in the bladder by cystoscopy, biopsy [if indicated], and urine cytology) and duration of response.
The median age of patients was 70 years (range, 30-92 years); 62% were male; race was White (97.8%), Black (0.9%), Asian (0.9%), or not reported (0.4%); 1.3% were Hispanic/Latino. Multiple tumors were present in 84% of patients, 6% had a tumor > 3 cm, 55% had a previous LG-NMIBC occurrence within 1 year of the current diagnosis, and all patients had a prior TURBT for LG-NMIBC.
Efficacy results are summarized in Table 3.
Table 3: Efficacy Results in ENVISION Efficacy Outcome Measure ZUSDURI
N = 223+ Denotes ongoing response. - * Based on observed duration of response for 173 patients who had a complete response.
Complete Response Rate %
(95% CI)78%
(72, 83)Duration of Response* Range in months (0.0, 25.0+) % (n) with duration ≥ 12 months 79% (137) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ZUSDURI is available in a kit (NDC: 72493-106-03) containing the following:
- Two 40 mg (each) single-dose vials of mitomycin for intravesical solution supplied as a sterile, lyophilized, grey to greyish-purple, cake or powder. (NDC: 72493-104-40)
- One single-dose vial of 60 mL sterile hydrogel supplied as a sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution. (NDC: 72493-105-60)
Storage and Handling
Store ZUSDURI at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Avoid excessive heat over 40°C (104°F). Protect from light.
ZUSDURI is a hazardous drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ZUSDURI and for 6 months following the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZUSDURI and for 3 months following the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with ZUSDURI and for 1 week following the last dose [see Use in Specific Populations (8.2)].
Important Post-Treatment Instructions [see Dosage and Administration (2.4)]
Advise patients that ZUSDURI contains mitomycin which is a violet to blue color and may discolor urine following the instillation procedure.
Advise patients to avoid contact with urine for at least 24 hours post-instillation [see Clinical Pharmacology (12.3)].
Advise patients to avoid urine contact with skin by voiding sitting on a toilet, flushing the toilet several times after use, and to wash hands, perineum or glans with soap and water after each instillation procedure.
Advise patients to wash clothing soiled with urine promptly and separately from other clothing.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
ZUSDURI™ (zus-dur-ee)
(mitomycin)
for intravesical solutionThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 06/2025 What is ZUSDURI?
ZUSDURI is a prescription medicine used to treat adults with a type of cancer of the lining of the bladder called low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC) after you have previously received bladder surgery to remove tumor and it did not work or is no longer working.
It is not known if ZUSDURI is safe and effective for use in children.Who should not receive ZUSDURI?
Do not receive ZUSDURI if you:- have a hole or tear (perforation) of your bladder,
- have had an allergic reaction to mitomycin or to any of the ingredients in ZUSDURI. See the end of this Patient Information leaflet for a complete list of the ingredients in ZUSDURI.
Before receiving ZUSDURI, tell your healthcare provider about all of your medical conditions, including if you: - have kidney problems.
- are pregnant or plan to become pregnant. ZUSDURI can harm your unborn baby. You should not become pregnant during treatment with ZUSDURI. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ZUSDURI.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before starting treatment with ZUSDURI.
- You should use effective birth control (contraception) during treatment with ZUSDURI and for 6 months after the last dose.
- Talk to your healthcare provider if you have questions about birth control options that are right for you.
- If you have a female partner who is able to become pregnant, you should use effective birth control (contraception) during treatment with ZUSDURI and for 3 months after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if ZUSDURI passes into your breast milk. Do not breastfeed during treatment with ZUSDURI and for 1 week after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. How will I receive ZUSDURI? - ZUSDURI will be given to you by your healthcare provider.
- You will receive ZUSDURI 1 time a week for 6 weeks into your bladder through a tube called a urinary catheter. It is important that you receive all 6 doses of ZUSDURI according to your healthcare provider's instructions.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
- During treatment with ZUSDURI, your healthcare provider may tell you to take additional medicines or change how you take your current medicines. Ask your healthcare provider if you have any questions.
- ZUSDURI may cause your urine color to change to a violet to blue color.
- Avoid contact between your skin and urine for at least 24 hours.
- To urinate, males and females should sit on a toilet and flush the toilet several times after you use it.
- After going to the bathroom, wash your hands, your inner thighs, and genital area well with soap and water.
- Clothing that comes in contact with urine should be washed right away and washed separately from other clothing.
What are the possible side effects of ZUSDURI? The most common side effects of ZUSDURI include: - increased blood creatinine levels
- increased blood potassium levels
- trouble with urination
- decreased red blood cell counts
- increase in certain blood liver tests
- increased or decreased white blood cell counts
- urinary tract infection
- blood in your urine
Call your doctor for medical advice about possible side effects. You may report side effects to FDA at 1-800-FDA-1088.
You can also report side effects to UroGen Pharma at 1-855-987-6436.General information about ZUSDURI.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about ZUSDURI that is written for healthcare professionals.What are the ingredients of ZUSDURI?
Active ingredient: mitomycin
Inactive ingredients: hydroxypropyl methylcellulose, mannitol, poloxamer, polyethylene glycol, and water for injection
Distributed by: UroGen Pharma, Inc., Princeton, NJ 08540
ZUSDURI™ is a trademark and UroGen® is a registered trademark of UroGen Pharma, Ltd.
U.S. Patent Nos. 9,040,074, 9,950,069 and 10,039,832
Copyright© 2025 UroGen Pharma, Inc.
All rights reserved.
For more information go to www.ZUSDURI.com or call 1-855-987-6436.ZUS-PPI-001
-
INSTRUCTIONS FOR PHARMACY (IFP) ZUSDURI™ (zus-dur-ee) (mitomycin) for intravesical solution
Purpose of this Instructions for Pharmacy
This Instructions for Pharmacy contains information on how to prepare ZUSDURI using pharmacy supplies and a Chilling Block or other means of chilling.
Intended Use of ZUSDURI
ZUSDURI (mitomycin) for intravesical solution is indicated for the treatment of adult patients with recurrent low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
Important Information You Need to Know Before Reconstituting ZUSDURI
Reconstituted ZUSDURI must be prepared under chilled conditions. ZUSDURI cannot be prepared without a Chilling Block or other means of chilling.
Once reconstituted with sterile hydrogel, ZUSDURI will appear as a semisolid gel under room temperature conditions and as a viscous liquid under chilled conditions.
Preparation of ZUSDURI must be performed under aseptic conditions.
Storage Conditions and Handling
Before reconstitution, store ZUSDURI at room temperature, 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C and 30°C (59°F and 86°F). Avoid excessive heat over 40°C (104°F). Protect from light.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
ZUSDURI is a hazardous drug. Procedures for Proper Handling and Disposal of hazardous drugs should be followed.
Supplies Needed
ZUSDURI available in a kit containing: - Two vials of ZUSDURI, 40 mg per vial
- One vial of Sterile hydrogel, 60 mL
- One ZUSDURI admixture label
- ZUSDURI Prescribing Information (PI)
- ZUSDURI Instructions for Pharmacy (IFP)
- ZUSDURI Instructions for Administration (IFA)
Pharmacy Supplies:
(Provided by your facility)
 Do not substitute any of these components
Do not substitute any of these components
Two 20 mL Luer lock syringes 
Two 5 mL Luer lock syringes filled with 3 mL sterile water 
Three OnGuard®2 CSTD vial adaptors 
Four OnGuard®2 CSTD syringe adaptors 
Chilling Block or other means of chilling (device design may vary)
Note: The day before the preparation, place the Chilling Block in the freezer at -20°C to -12°C (-4°F to 10.4°F). Refer to the Chilling Block Instructions for Use for more information.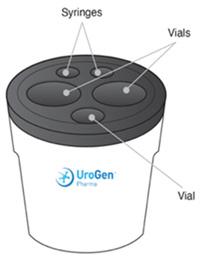
Steps to Prepare ZUSDURI Admixture
- A. Freeze the Chilling Block
- 1. The day before preparation, put the Chilling Block in the freezer at -20°C to -12°C (-4°F to 10.4°F).
Note: Please refer to the Chilling Block Instructions for Use for additional information.- B. Prepare Supplies
- 1. Remove the Chilling Block from the freezer.
- 2. Disinfect the Chilling Block as per your pharmacy's policy. Allow it to air dry, and then place it inside the hood or isolator.
- 3. Connect vial adaptors to all three vials.
- 4. Connect syringe adaptors to both empty 20 mL syringes and both sterile water syringes.
- 5. Place one ZUSDURI vial, the sterile hydrogel vial, and both empty 20 mL syringes in the Chilling Block for approximately 20 minutes.
Note: The Chilling Block has five compartments, but only four are needed. The small vial compartment will not be used during this preparation.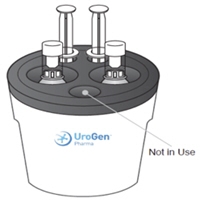
- 6. Set the second ZUSDURI vial and both sterile water syringes aside for later use.
- C. Reconstitute the First ZUSDURI Vial
- 1. Slowly fill one chilled 20 mL syringe with 14 mL of sterile hydrogel.
- 2. Slowly fill the other chilled 20 mL syringe with 13 mL of sterile hydrogel.
- 3. Place both 20 mL syringes and the sterile hydrogel vial back in the Chilling Block.
- 4. Remove the chilled ZUSDURI vial from the Chilling Block.
- 5. Inject 3 mL of sterile water into the ZUSDURI vial.
- 6. Gently swirl the ZUSDURI vial upright at least 20 times.
Note: Do not invert or shake the vial.- 7. Inject the chilled 14 mL and 13 mL syringes of sterile hydrogel into the ZUSDURI vial.
- 8. Place both empty 20 mL syringes back in the Chilling Block for later use.
- 9. Vigorously swirl the ZUSDURI vial upright for about 2 minutes.
Note: Do not invert or shake the vial.- 10. Set the first ZUSDURI vial aside for later use.
- D. Reconstitute the Second ZUSDURI Vial
- 1. Place the second ZUSDURI vial in the Chilling Block.
- 2. Slowly fill one chilled 20 mL syringe with 14 mL of sterile hydrogel.
- 3. Slowly fill the other chilled 20 mL syringe with 13 mL of sterile hydrogel.
- 4. Place both 20 mL syringes back in the Chilling Block.
- 5. Discard the empty sterile hydrogel vial.
- 6. Remove the chilled ZUSDURI Vial from the Chilling Block.
- 7. Inject 3 mL of sterile water into the ZUSDURI vial.
- 8. Gently swirl the ZUSDURI vial upright at least 20 times.
Note: Do not invert or shake the vial.- 9. Inject the chilled 14 mL and 13 mL syringes of sterile hydrogel into the ZUSDURI vial.
- 10. Place both empty 20 mL syringes back in the Chilling Block for later use.
- 11. Vigorously swirl the ZUSDURI vial upright for about 2 minutes.
Note: Do not invert or shake the vial.- E. Combine the Contents of the ZUSDURI Admixture Vials
- 1. Slowly fill each chilled 20 mL syringe with 14 mL of admixture from the second ZUSDURI vial (for a total of 28 mL).
Note: If you are having difficulty withdrawing the admixture, place the components back in the Chilling Block until the admixture liquifies again.- 2. Inject the two 14 mL admixture syringes into the first ZUSDURI vial.
- 3. You now have one vial that contains ZUSDURI admixture.
- F. Dispense Reconstituted ZUSDURI
- 1. Write the "Discard after" date and time on the supplied admixture label and apply to the prepared ZUSDURI admixture vial. You may also substitute your pharmacy's label for dispensing the ZUSDURI admixture.
Note: The "Discard after" date and time is 7 days from the completion of the preparation. Store reconstituted ZUSDURI at 2°C to 8°C (36°F to 46°F) for up to 7 days. Alternatively, reconstituted ZUSDURI may be stored under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F). Avoid excessive heat over 40°C (104°F). Protect from light.- 2. Transport the following items to the treatment facility:
- i. One reconstituted ZUSDURI vial.
- ii. ZUSDURI Instructions for Administration (IFA).
 Important to Remember
Important to Remember
- All components need to be kept cold during the preparation process by placing them in the Chilling Block or other means of chilling when they are not in use.
- If you are having difficulty pushing or withdrawing the solution at any time, place the components back in the Chilling Block or other means of chilling until the product liquifies again.
Frequently Asked Questions
This Instructions for Pharmacy has been approved by the U.S. Food and Drug Administration. How do I connect the vial adaptor?
Place the vial adaptor over the vial and press down firmly. You will hear a snap which confirms successful attachment.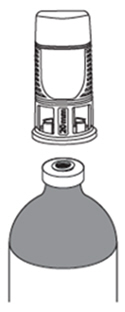
How do I connect the syringe adaptor?
Screw the Luer lock end of the syringe adaptor onto the syringe hub until it is finger tight.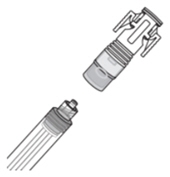
How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs, then press down firmly. You will hear a snap which confirms successful attachment.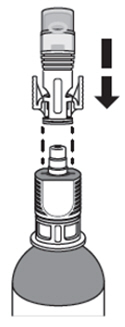
How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor.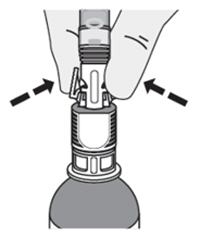
Do I have to keep the vials upright when mixing? If so, why?
Yes, the vials should be kept upright when mixing to ensure the contents are fully mixed at the bottom of the vial.
I am having difficulty working with the drug product. It seems to be solidifying.
If you are having difficulty pushing or withdrawing the solution, place the components back in the Chilling Block or other means of chilling until it liquifies. - SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR ADMINISTRATION (IFA) ZUSDURI™ (zus-dur-ee) (mitomycin) for intravesical solution For Intravesical Instillation Only

Read and follow this Instructions for Administration prior to each ZUSDURI instillation. Purpose of this Instructions for Administration
This Instructions for Administration contains information on how to instill ZUSDURI using the ZUSDURI vial and the devices listed under Supplies Needed, obtained by your facility.
Intended Use of ZUSDURI
ZUSDURI (mitomycin) for intravesical solution is indicated for the treatment of adult patients with recurrent low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
Important Information You Need to Know Before Instilling ZUSDURI
ZUSDURI must be reconstituted by a healthcare professional prior to instillation. Reconstituted ZUSDURI will appear as a semisolid gel. Once chilled, ZUSDURI will convert to a viscous liquid for instillation.
Once instilled into the patient's bladder, ZUSDURI will convert to a semisolid gel, thereby exposing the lining of the bladder to mitomycin over a prolonged period of time.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
When ready to instill, chill ZUSDURI at -3°C to 5°C (27°F to 41°F) for about 20 minutes to revert it to a viscous liquid. See Steps A through C below for complete administration instructions.
ZUSDURI is a hazardous drug. Procedures for proper handling and disposal of hazardous drugs should be followed, as described below.
Supplies Needed
One vial of reconstituted ZUSDURI, with resultant concentration of 80 mg per 60 mL.
(prepared and provided by your pharmacy)
Ice Bath
Note: The ice bath should consist of ice and cold water and be large enough to submerge a ZUSDURI vial.
Plastic Bag 
Ancillary Supplies
When you administer ZUSDURI, you will need to use special adaptors for your catheter and syringes. These adaptors will create a closed pathway to protect you and your patient during and after administration. Fluid will only be able to pass between connection points when the adaptors are fully engaged, preventing drips or leaks.
Once the adaptors are connected, do not disconnect them. They are there for your protection.
Urinary Catheter
One 12 Fr to 16 Fr with fixed Luer lock connector
 Do not substitute
Do not substitute
Catheter Adaptor
One OnGuard®2 CSTD Luer lock adaptor
 Do not substitute
Do not substitute
Administration Syringes
Four 20 mL polypropylene Luer lock syringes (BD Luer-Lok™ Syringe)
 Do not substitute
Do not substitute
Sterile Water Flush
One 5 mL Luer lock syringe filled with 3 mL sterile water
Syringe Adaptors
Five OnGuard®2 CSTD syringe adaptors
 Do not substitute
Do not substitute
Instillation Instructions
- A. Chill the ZUSDURI
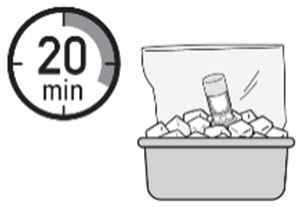
- 1. Place the ZUSDURI vial in a plastic bag.
- 2. Place the plastic bag in the ice bath for approximately 20 minutes.
Note: Ensure the ZUSDURI vial is completely immersed in the ice bath.
- B. Prepare the Four Administration Syringes
- 1. Connect a syringe adaptor to the 3 mL sterile water syringe and set aside.
- 2. Connect syringe adaptors to the four 20 mL syringes. These will be the administration syringes.
Note: Once the adaptors are connected, do not disconnect them. They are there for your protection.- 3. Remove the ZUSDURI vial from the ice bath and verify the admixture is liquid. If the admixture is not liquid, place the vial bag back into the ice bath until the admixture liquefies.
- 4. Swirl the vial upright to ensure that ZUSDURI is uniformly mixed.
- 5. Slowly fill each of the four administration syringes with 14 mL of ZUSDURI admixture (for a total of 56 mL).
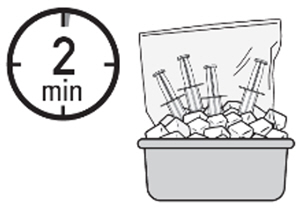
Note: there might be foaming in the syringe while withdrawing ZUSDURI.- 6. Place the four administration syringes in the plastic bag.
- 7. Place the plastic bag in the ice bath, ensuring the four administration syringes are completely immersed, for at least two minutes.
- C. Instill ZUSDURI
 The contents of four administration syringes must be instilled in rapid succession without delay to avoid ZUSDURI solidification. Before proceeding:
The contents of four administration syringes must be instilled in rapid succession without delay to avoid ZUSDURI solidification. Before proceeding:- Ensure that all four administration syringes contain liquid ZUSDURI.
- Keep all four administration syringes chilled in the ice bath until instillation time.
- 1. Place the urinary catheter in the bladder and drain the urine.
- 2. Connect the catheter adaptor to the urinary catheter.
- 3. Connect an administration syringe to the urinary catheter.
- 4. Instill the ZUSDURI into the patient.
Note: Empty each administration syringe over approximately 30 seconds. ZUSDURI is viscous, even in a chilled state.
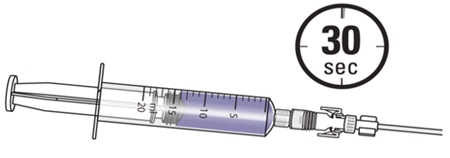
- 5. Disconnect the syringe adaptor from the catheter adaptor.
- 6. Immediately repeat these steps without delay for the remaining three administration syringes.
- 7. After all four administration syringes have been instilled, flush the urinary catheter with 3 mL of sterile water.
- 8. Wait 15 minutes before removing the urinary catheter from the patient.

- 9. Discard all ancillary supplies according to your facility's procedures for hazardous waste.
Frequently Asked Questions
How do I connect the syringe adaptor?
Screw the Luer lock end of the syringe adaptor onto the syringe hub until it is finger tight.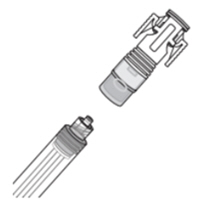
How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs; then press down firmly. You will hear a snap which confirms successful attachment.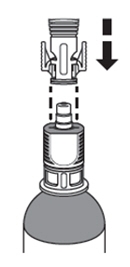
How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor.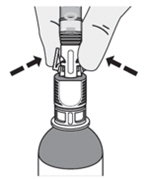
I am having a hard time working with ZUSDURI. It seems to be solidifying.
If you are having difficulty pushing or withdrawing ZUSDURI, recap and place the components back into the ice bath until ZUSDURI liquifies. The vial may be placed upside down within the bag in the ice bath.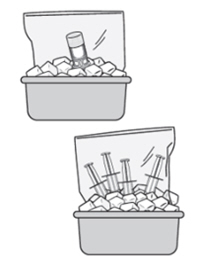
How do I connect the catheter adaptor to the urinary catheter?
Screw the Luer lock end of the catheter adaptor onto the urinary catheter connector until it is finger tight.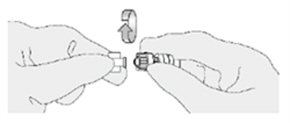
How do I connect the syringe adaptor to the catheter adaptor?
Place the syringe adaptor in front of the catheter adaptor and push it on firmly. You will hear a snap which confirms successful attachment.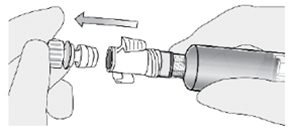
How do I disconnect the syringe adaptor from the catheter adaptor?
While holding the urinary catheter steady and fixed in its position, pinch the tabs on the syringe adaptor to separate it from the catheter adaptor.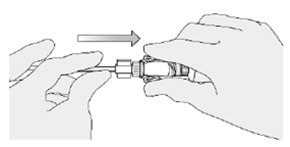
This "Instructions for Administration" has been approved by the U.S. Food and Drug Administration.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
www.ZUSDURI.comZUSDURI™ is a trademark of UroGen Pharma, Ltd.
OnGuard® is a registered trademark of Simplivia Healthcare Ltd.
BD Luer-Lok™ is a trademark of Becton, Dickinson and Company.Copyright© 2025 UroGen Pharma, Inc.
All rights reserved.ZUS-IFA-001
IFA-0017644/Ver. 1 -
PRINCIPAL DISPLAY PANEL - 40 mg Kit Carton
NDC: 72493-106-03
Zusduri™
(mitomycin) for intravesical solution
40 mg per VialAttention Pharmacist: Reconstitution
is required prior to dispensing.
See the Instructions for Pharmacy
before proceeding.SINGLE-DOSE KIT
Warning: For Intravesical Use OnlyRx Only
Contents of Kit:
- 2 Single-Dose Vials of ZUSDURI (mitomycin) for intravesical solution, 40 mg per Vial.
- 1 Single-Dose Vial of Sterile Hydrogel, 60 mL per Vial
- 1 Admixture Label
- Full Prescribing Information
- Instructions for Pharmacy
- Instructions for Administration
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F). Protect from light.
Store reconstituted ZUSDURI at 2°C to 8°C (36°F to 46°F) for up to 7 days.
Alternatively, reconstituted ZUSDURI may be stored under refrigeration at 2°C to 8°C (36°F to 46°F)
for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
Avoid excessive heat over 40°C (104°F).
Protect reconstituted ZUSDURI from light.
When ready to instill, chill ZUSDURI at -3°C to 5°C (27°F to 41°F) for about 20 minutes to revert it to a
viscous liquid.Recommended Dosage: See Full Prescribing Information.
Warning: Hazardous Drug
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540UroGen®
Pharma
-
PRINCIPAL DISPLAY PANEL - 40 mg Vial Label
NDC: 72493-104-40
Single-Dose Vial
SterileZusduri™
(mitomycin) for intravesical solution
40 mg per VialSee Instructions for Pharmacy for preparation instructions
Must be Reconstituted with Sterile Hydrogel Before UseWarning: For Intravesical Use Only
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C
(59°F and 86°F). Avoid excessive heat over 40°C (104°F). Protect from light.
Each vial contains: Mitomycin 40 mg and mannitol 80 mg.Recommended Dosage: See Full Prescribing Information.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540Lot
ExpWarning: Hazardous Drug
Rx OnlyUroGen®
Pharma620565
LBL-0017642/Ver. 1

-
PRINCIPAL DISPLAY PANEL - 60 mL Vial Label
NDC: 72493-105-60
Single-Dose VialSterile Hydrogel
For use in preparation of ZUSDURI™
(mitomycin) for intravesical solutionNot for Direct Administration
See Instructions for Pharmacy for
preparation instructionsStore at 20°C to 25°C (68°F to 77°F); excursions
permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F).Rx Only
60 mL per Vial
LBL-0017641/Ver. 1

-
INGREDIENTS AND APPEARANCE
ZUSDURI
mitomycin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72493-106 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-106-03 1 in 1 CARTON 07/01/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, SINGLE-DOSE 2 Part 2 1 VIAL, SINGLE-DOSE 60 mL Part 1 of 2 ZUSDURI
mitomycin powder, for solutionProduct Information Item Code (Source) NDC: 72493-104 Route of Administration INTRAVESICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mitomycin (UNII: 50SG953SK6) (Mitomycin - UNII:50SG953SK6) Mitomycin 40 mg Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-104-40 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215793 07/01/2025 Part 2 of 2 ZUSDURI
sterile hydrogel gelProduct Information Item Code (Source) NDC: 72493-105 Route of Administration INTRAVESICAL Inactive Ingredients Ingredient Name Strength POLOXAMER 407 (UNII: TUF2IVW3M2) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-105-60 60 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215793 07/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215793 07/01/2025 Labeler - UroGen Pharma, Inc (081130487) Registrant - UroGen Pharma, Ltd. (534155213)
Trademark Results [ZUSDURI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZUSDURI 90628285 not registered Live/Pending |
UroGen Pharma Ltd. 2021-04-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.