TAMOXIFEN CITRATE tablet, film coated
Tamoxifen Citrate by
Drug Labeling and Warnings
Tamoxifen Citrate by is a Prescription medication manufactured, distributed, or labeled by Mayne Pharma Inc., Teva Pharmaceutical Industries Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
For Women With Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer
Serious and life-threatening events associated with tamoxifen in the risk reduction setting (women at high risk for cancer and women with DCIS) include uterine malignancies, stroke and pulmonary embolism. Incidence rates for these events were estimated from the NSABP P-1 trial (see CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women). Uterine malignancies consist of both endometrial adenocarcinoma (incidence rate per 1,000 women-years of 2.20 for tamoxifen vs. 0.71 for placebo) and uterine sarcoma (incidence rate per 1,000 women-years of 0.17 for tamoxifen vs. 0.4 for placebo)*. For stroke, the incidence rate per 1,000 women-years was 1.43 for tamoxifen vs. 1.00 for placebo**. For pulmonary embolism, the incidence rate per 1,000 women-years was 0.75 for tamoxifen versus 0.25 for placebo**.
Some of the strokes, pulmonary emboli, and uterine malignancies were fatal.
Health care providers should discuss the potential benefits versus the potential risks of these serious events with women at high risk of breast cancer and women with DCIS considering tamoxifen to reduce their risk of developing breast cancer.
The benefits of tamoxifen outweigh its risks in women already diagnosed with breast cancer.
* Updated long-term follow-up data (median length of follow-up is 6.9 years) from NSABP P-1 study. See WARNINGS, Effects on the Uterus-Endometrial Cancer and Uterine Sarcoma.
** See Table 3 under CLINICAL PHARMACOLOGY, Clinical Studies.
-
DESCRIPTION
Tamoxifen citrate tablets USP, a nonsteroidal antiestrogen, are for oral administration. Each tablet contains 10 mg or 20 mg tamoxifen (equivalent to 15.2 mg or 30.4 mg, respectively, of tamoxifen citrate, USP).
Each tablet contains the following inactive ingredients: croscarmellose sodium, hypromellose, lactose (monohydrate), magnesium stearate, polyethylene glycol 400, povidone, corn starch, and titanium dioxide.
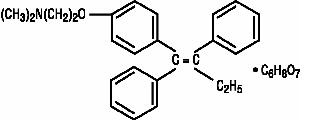
Chemically, tamoxifen is the trans-isomer of a triphenylethylene derivative. The chemical name is (Z)2-[4-(1,2-diphenyl-1-butenyl)phenoxy]- N,N-dimethylethanamine 2-hydroxy-1,2,3- propanetricarboxylate (1:1). The structural formula, empirical formula, and molecular weight are as follows:
C32H37NO8 M.W. 563.62
Tamoxifen citrate has a pKa' of 8.85, the equilibrium solubility in water at 37°C is 0.5 mg/mL and in 0.02 N HCl at 37°C, it is 0.2 mg/mL.
-
CLINICAL PHARMACOLOGY
Tamoxifen citrate is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Tamoxifen inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, tamoxifen appears to exert its antitumor effects by binding the estrogen receptors.
In cytosols derived from human breast adenocarcinomas, tamoxifen competes with estradiol for estrogen receptor protein.
Absorption and Distribution
Following a single oral dose of 20 mg tamoxifen, an average peak plasma concentration of 40 ng/mL (range 35 to 45 ng/mL) occurred approximately 5 hours after dosing. The decline in plasma concentrations of tamoxifen is biphasic with a terminal elimination half-life of about 5 to 7 days. The average peak plasma concentration of N-desmethyl tamoxifen is 15 ng/mL (range 10 to 20 ng/mL). Chronic administration of 10 mg tamoxifen given twice daily for 3 months to patients results in average steady-state plasma concentrations of 120 ng/mL (range 67 to 183 ng/mL) for tamoxifen and 336 ng/mL (range 148 to 654 ng/mL) for N-desmethyl tamoxifen. The average steady-state plasma concentrations of tamoxifen and N-desmethyl tamoxifen after administration of 20 mg tamoxifen once daily for 3 months are 122 ng/mL (range 71 to 183 ng/mL) and 353 ng/mL (range 152 to 706 ng/mL), respectively. After initiation of therapy, steady-state concentrations for tamoxifen are achieved in about 4 weeks and steady-state concentrations for N-desmethyl tamoxifen are achieved in about 8 weeks, suggesting a half-life of approximately 14 days for this metabolite. In a steady-state, crossover study of 10 mg tamoxifen citrate tablets given twice a day vs. a 20 mg tamoxifen citrate tablet given once daily, the 20 mg tamoxifen citrate tablet was bioequivalent to the 10 mg tamoxifen citrate tablets.
Metabolism
Tamoxifen is extensively metabolized after oral administration. N-desmethyl tamoxifen is the major metabolite found in patients' plasma. The biological activity of N-desmethyl tamoxifen appears to be similar to that of tamoxifen. 4-Hydroxytamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. Tamoxifen is a substrate of cytochrome P-450 3A, 2C9 and 2D6, and an inhibitor of P-glycoprotein.
Excretion
Studies in women receiving 20 mg of 14C tamoxifen have shown that approximately 65% of the administered dose was excreted from the body over a period of 2 weeks with fecal excretion as the primary route of elimination. The drug is excreted mainly as polar conjugates, with unchanged drug and unconjugated metabolites accounting for less than 30% of the total fecal radioactivity.
Special Populations
The effects of age, gender and race on the pharmacokinetics of tamoxifen have not been determined. The effects of reduced liver function on the metabolism and pharmacokinetics of tamoxifen have not been determined.
Pediatric Patients
The pharmacokinetics of tamoxifen and N-desmethyl tamoxifen were characterized using a population pharmacokinetic analysis with sparse samples per patient obtained from 27 female pediatric patients aged 2 to 10 years enrolled in a study designed to evaluate the safety, efficacy, and pharmacokinetics of tamoxifen in treating McCune-Albright syndrome. Rich data from two tamoxifen citrate pharmacokinetic trials in which 59 postmenopausal women with breast cancer completed the studies were included in the analysis to determine the structural pharmacokinetic model for tamoxifen. A one-compartment model provided the best fit to the data.
In pediatric patients, an average steady-state peak plasma concentration (Css, max) and AUC were of 187 ng/mL and 4,110 ng hr/mL, respectively, and Css, max occurred approximately 8 hours after dosing. Clearance (CL/F) as body weight adjusted in female pediatric patients was approximately 2.3 fold higher than in female breast cancer patients. In the youngest cohort of female pediatric patients (2 to 6 year olds), CL/F was 2.6 fold higher; in the oldest cohort (7 to 10.9 year olds) CL/F was approximately 1.9 fold higher. Exposure to N-desmethyl tamoxifen was comparable between the pediatric and adult patients. The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy in girls have not been established. In adults treated with tamoxifen an increase in incidence of uterine malignancies, stroke and pulmonary embolism has been noted (see BOXED WARNING).
Drug-Drug Interactions
In vitro studies showed that erythromycin, cyclosporin, nifedipine and diltiazem competitively inhibited formation of N-desmethyl tamoxifen with apparent K1 of 20, 1, 45 and 30 µM, respectively. The clinical significance of these in vitro studies is unknown.
Tamoxifen reduced the plasma concentration of letrozole by 37% when these drugs were coadministered. Rifampin, a cytochrome P-450 3A4 inducer reduced tamoxifen AUC and Cmax by 86% and 55%, respectively. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
In the anastrozole adjuvant trial, coadministration of anastrozole and tamoxifen in breast cancer patients reduced anastrozole plasma concentration by 27% compared to those achieved with anastrozole alone; however, the coadministration did not affect the pharmacokinetics of tamoxifen or N-desmethyltamoxifen (see PRECAUTIONS, Drug Interactions). Tamoxifen should not be coadministered with anastrozole.
Clinical Studies
Metastatic Breast Cancer
Premenopausal women (tamoxifen vs. ablation)
Three prospective, randomized studies (Ingle, Pritchard, Buchanan) compared tamoxifen to ovarian ablation (oophorectomy or ovarian irradiation) in premenopausal women with advanced breast cancer. Although the objective response rate, time to treatment failure, and survival were similar with both treatments, the limited patient accrual prevented a demonstration of equivalence. In an overview analysis of survival data from the 3 studies, the hazard ratio for death (tamoxifen/ovarian ablation) was 1.00 with two-sided 95% confidence intervals of 0.73 to 1.37. Elevated serum and plasma estrogens have been observed in premenopausal women receiving tamoxifen, but the data from the randomized studies do not suggest an adverse effect of this increase. A limited number of premenopausal patients with disease progression during tamoxifen therapy responded to subsequent ovarian ablation.
Male breast cancer
Published results from 122 patients (119 evaluable) and case reports in 16 patients (13 evaluable) treated with tamoxifen have shown that tamoxifen is effective for the palliative treatment of male breast cancer. Sixty-six of these 132 evaluable patients responded to tamoxifen which constitutes a 50% objective response rate.
Adjuvant Breast Cancer
Overview
The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) conducted worldwide overviews of systemic adjuvant therapy for early breast cancer in 1985, 1990, and again in 1995. In 1998, 10 year outcome data were reported for 36,689 women in 55 randomized trials of adjuvant tamoxifen using doses of 20 to 40 mg/day for 1 to 5+ years. Twenty-five percent of patients received 1 year or less of trial treatment, 52% received 2 years, and 23% received about 5 years. Forty-eight percent of tumors were estrogen receptor (ER) positive (> 10 fmol/mg), 21% were ER poor (< 10 fmol/l), and 31% were ER unknown. Among 29,441 patients with ER positive or unknown breast cancer, 58% were entered into trials comparing tamoxifen to no adjuvant therapy and 42% were entered into trials comparing tamoxifen in combination with chemotherapy vs. the same chemotherapy alone. Among these patients, 54% had node positive disease and 46% had node negative disease.
Among women with ER positive or unknown breast cancer and positive nodes who received about 5 years of treatment, overall survival at 10 years was 61.4% for tamoxifen vs. 50.5% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 59.7% for tamoxifen vs. 44.5% for control (logrank 2p < 0.00001). Among women with ER positive or unknown breast cancer and negative nodes who received about 5 years of treatment, overall survival at 10 years was 78.9% for tamoxifen vs. 73.3% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 79.2% for tamoxifen vs. 64.3% for control (logrank 2p < 0.00001).
The effect of the scheduled duration of tamoxifen may be described as follows. In women with ER positive or unknown breast cancer receiving 1 year or less, 2 years or about 5 years of tamoxifen, the proportional reductions in mortality were 12%, 17% and 26%, respectively (trend significant at 2p < 0.003). The corresponding reductions in breast cancer recurrence were 21%, 29% and 47% (trend significant at 2p < 0.00001).
Benefit is less clear for women with ER poor breast cancer in whom the proportional reduction in recurrence was 10% (2p = 0.007) for all durations taken together, or 9% (2p = 0.02) if contralateral breast cancers are excluded. The corresponding reduction in mortality was 6% (NS). The effects of about 5 years of tamoxifen on recurrence and mortality were similar regardless of age and concurrent chemotherapy. There was no indication that doses greater than 20 mg per day were more effective.
Anastrozole adjuvant ATAC trial – study of anastrozole compared to tamoxifen for adjuvant treatment of early breast cancer
An anastrozole adjuvant trial was conducted in 9,366 postmenopausal women with operable breast cancer who were randomized to receive adjuvant treatment with either anastrozole 1 mg daily, tamoxifen 20 mg daily, or a combination of these two treatments for 5 years or until recurrence of the disease. At a median follow-up of 33 months, the combination of anastrozole and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen therapy alone in all patients as well as in the hormone receptor-positive subpopulation. This treatment arm was discontinued from the trial. Please refer to CLINICAL PHARMACOLOGY, Special Populations and Drug-Drug Interactions; PRECAUTIONS, Laboratory Tests; PRECAUTIONS, Drug Interactions and ADVERSE REACTIONS for safety information from this trial. Please refer to the full prescribing information for anastrozole 1 mg tablets for additional information on this trial.
Patients in the two monotherapy arms of the ATAC trial were treated for a median of 60 months (5 years) and followed for a median of 68 months. Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.87, 95% CI: 0.78, 0.97, p = 0.0127] in the anastrozole arm compared to the tamoxifen arm.
Node positive – individual studies
Two studies (Hubay and NSABP B-09) demonstrated an improved disease-free survival following radical or modified radical mastectomy in postmenopausal women or women 50 years of age or older with surgically curable breast cancer with positive axillary nodes when tamoxifen was added to adjuvant cytotoxic chemotherapy. In the Hubay study, tamoxifen was added to "low-dose" CMF (cyclophosphamide, methotrexate and fluorouracil). In the NSABP B-09 study, tamoxifen was added to melphalan [L-phenylalanine mustard (P)] and fluorouracil (F).
In the Hubay study, patients with a positive (more than 3 fmol) estrogen receptor were more likely to benefit. In the NSABP B-09 study in women age 50 to 59 years, only women with both estrogen and progesterone receptor levels 10 fmol or greater clearly benefited, while there was a nonstatistically significant trend toward adverse effect in women with both estrogen and progesterone receptor levels less than 10 fmol. In women age 60 to 70 years, there was a trend toward a beneficial effect of tamoxifen without any clear relationship to estrogen or progesterone receptor status.
Three prospective studies (ECOG-1178, Toronto, NATO) using tamoxifen adjuvantly as a single agent demonstrated an improved disease-free survival following total mastectomy and axillary dissection for postmenopausal women with positive axillary nodes compared to placebo/no treatment controls. The NATO study also demonstrated an overall survival benefit.
Node negative – individual studies
NSABP B-14, a prospective, double-blind, randomized study, compared tamoxifen to placebo in women with axillary node-negative, estrogen-receptor positive (≥ 10 fmol/mg cytosol protein) breast cancer (as adjuvant therapy, following total mastectomy and axillary dissection, or segmental resection, axillary dissection, and breast radiation). After five years of treatment, there was a significant improvement in disease-free survival in women receiving tamoxifen. This benefit was apparent both in women under age 50 and in women at or beyond age 50.
One additional randomized study (NATO) demonstrated improved disease-free survival for tamoxifen compared to no adjuvant therapy following total mastectomy and axillary dissection in postmenopausal women with axillary node-negative breast cancer. In this study, the benefits of tamoxifen appeared to be independent of estrogen receptor status.
Duration of therapy
In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy.
In the NSABP B-14 trial, in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo and were disease-free at the end of this 5 year period were offered rerandomization to an additional 5 years of tamoxifen or placebo. With 4 years of follow-up after this rerandomization, 92% of the women that received 5 years of tamoxifen were alive and disease-free, compared to 86% of the women scheduled to receive 10 years of tamoxifen (p = 0.003). Overall survivals were 96% and 94%, respectively (p = 0.08). Results of the B-14 study suggest that continuation of therapy beyond 5 years does not provide additional benefit.
A Scottish trial of 5 years of tamoxifen vs. indefinite treatment found a disease-free survival of 70% in the five-year group and 61% in the indefinite group, with 6.2 years median follow-up (HR = 1.27, 95% CI: 0.87 to 1.85).
In a large randomized trial conducted by the Swedish Breast Cancer Cooperative Group of adjuvant tamoxifen 40 mg/day for 2 or 5 years, overall survival at 10 years was estimated to be 80% in the patients in the 5 year tamoxifen group, compared with 74% among corresponding patients in the 2 year treatment group (p = 0.03). Disease-free survival at 10 years was 73% in the 5 year group and 67% in the 2 year group (p = 0.009). Compared with 2 years of tamoxifen treatment, 5 years of treatment resulted in a slightly greater reduction in the incidence of contralateral breast cancer at 10 years, but this difference was not statistically significant.
Contralateral breast cancer
The incidence of contralateral breast cancer is reduced in breast cancer patients (premenopausal and postmenopausal) receiving tamoxifen compared to placebo. Data on contralateral breast cancer are available from 32,422 out of 36,689 patients in the 1995 overview analysis of the Early Breast Cancer Trialists Collaborative Group (EBCTCG). In clinical trials with tamoxifen of 1 year or less, 2 years, and about 5 years duration, the proportional reductions in the incidence rate of contralateral breast cancer among women receiving tamoxifen were 13% (NS), 26% (2p = 0.004) and 47% (2p < 0.00001), with a significant trend favoring longer tamoxifen duration (2p = 0.008). The proportional reductions in the incidence of contralateral breast cancer were independent of age and ER status of the primary tumor. Treatment with about 5 years of tamoxifen reduced the annual incidence rate of contralateral breast cancer from 7.6 per 1,000 patients in the control group compared with 3.9 per 1,000 patients in the tamoxifen group.
In a large randomized trial in Sweden (the Stockholm Trial) of adjuvant tamoxifen 40 mg/day for 2 to 5 years, the incidence of second primary breast tumors was reduced 40% (p < 0.008) on tamoxifen compared to control. In the NSABP B-14 trial in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo, the incidence of second primary breast cancers was also significantly reduced (p < 0.01). In NSABP B-14, the annual rate of contralateral breast cancer was 8.0 per 1,000 patients in the placebo group compared with 5.0 per 1,000 patients in the tamoxifen group, at 10 years after first randomization.
Ductal Carcinoma in Situ
NSABP B-24, a double-blind, randomized trial included women with ductal carcinoma in situ (DCIS). This trial compared the addition of tamoxifen or placebo to treatment with lumpectomy and radiation therapy for women with DCIS. The primary objective was to determine whether 5 years of tamoxifen therapy (20 mg/day) would reduce the incidence of invasive breast cancer in the ipsilateral (the same) or contralateral (the opposite) breast.
In this trial 1,804 women were randomized to receive either tamoxifen or placebo for 5 years: 902 women were randomized to tamoxifen citrate 10 mg tablets twice a day and 902 women were randomized to placebo. As of December 31, 1998, follow-up data were available for 1,798 women and the median duration of follow-up was 74 months.
The tamoxifen and placebo groups were well balanced for baseline demographic and prognostic factors. Over 80% of the tumors were less than or equal to 1 cm in their maximum dimension, were not palpable, and were detected by mammography alone. Over 60% of the study population was postmenopausal. In 16% of patients, the margin of the resected specimen was reported as being positive after surgery. Approximately half of the tumors were reported to contain comedo necrosis.
For the primary endpoint, the incidence of invasive breast cancer was reduced by 43% among women assigned to tamoxifen (44 cases-tamoxifen, 74 cases-placebo; p = 0.004; relative risk (RR) = 0.57, 95% CI: 0.39 to 0.84). No data are available regarding the ER status of the invasive cancers. The stage distribution of the invasive cancers at diagnosis was similar to that reported annually in the SEER data base.
Results are shown in Table 1. For each endpoint the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.
Table 1: Major Outcomes of the NSABP B-24 Trial - * Updated follow-up data (median 8.1 years)
Type of Event
Lumpectomy, radiotherapy, and placebo
Lumpectomy, radiotherapy, and tamoxifen
RR
95% CI limits
No. of events
Rate per 1,000 women per year
No. of events
Rate per 1,000 women per year
Invasive breast cancer (Primary endpoint)
74
16.73
44
9.60
0.57
0.39 to 0.84
Ipsilateral
47
10.61
27
5.90
0.56
0.33 to 0.91
Contralateral
25
5.64
17
3.71
0.66
0.33 to 1.27
Side undetermined
2
--
0
--
--
Secondary Endpoints
DCIS
56
12.66
41
8.95
0.71
0.46 to 1.08
Ipsilateral
46
10.40
38
8.29
0.88
0.51 to 1.25
Contralateral
10
2.26
3
0.65
0.29
0.05 to 1.13
All Breast Cancer Events
129
29.16
84
18.34
0.63
0.47 to 0.83
All ipsilateral events
96
21.70
65
14.19
0.65
0.47 to 0.91
All contralateral events
37
8.36
20
4.37
0.52
0.29 to 0.92
Deaths
32
28
Uterine Malignancies*
4
9
Endometrial Adenocarcinoma*
4
0.57
8
1.15
Uterine Sarcoma*
0
0.0
1
0.14
Second primary malignancies (other than endometrial and breast)
30
29
Stroke
2
7
Thromboembolic events (DVT, PE)
5
15
Survival was similar in the placebo and tamoxifen groups. At 5 years from study entry, survival was 97% for both groups.
Reduction in Breast Cancer Incidence in High Risk Women
The Breast Cancer Prevention Trial (BCPT, NSABP P-1) was a double-blind, randomized, placebo-controlled trial with a primary objective to determine whether 5 years of tamoxifen therapy (20 mg/day) would reduce the incidence of invasive breast cancer in women at high risk for the disease (see INDICATIONS AND USAGE). Secondary objectives included an evaluation of the incidence of ischemic heart disease; the effects on the incidence of bone fractures; and other events that might be associated with the use of tamoxifen, including: endometrial cancer, pulmonary embolus, deep-vein thrombosis, stroke, and cataract formation and surgery (see WARNINGS).
The Gail Model was used to calculate predicted breast cancer risk for women who were less than 60 years of age and did not have lobular carcinoma in situ (LCIS). The following risk factors were used: age; number of first-degree female relatives with breast cancer; previous breast biopsies; presence or absence of atypical hyperplasia; nulliparity; age at first live birth; and age at menarche. A 5 year predicted risk of breast cancer of ≥ 1.67% was required for entry into the trial.
In this trial, 13,388 women of at least 35 years of age were randomized to receive either tamoxifen or placebo for five years. The median duration of treatment was 3.5 years. As of January 31, 1998, follow-up data is available for 13,114 women. Twenty-seven percent of women randomized to placebo (1,782) and 24% of women randomized to tamoxifen (1,596) completed 5 years of therapy. The demographic characteristics of women on the trial with follow-up data are shown in Table 2.
Table 2: Demographic Characteristics of Women in the NSABP P-1 Trial Characteristic
Placebo
Tamoxifen
#
%
#
%
Age (yrs.)
35 to 39
184
3
158
2
40 to 49
2,394
36
2,411
37
50 to 59
2,011
31
2,019
31
60 to 69
1,588
24
1,563
24
≥ 70
393
6
393
6
Age at first live birth (yrs.)
Nulliparous
1,202
18
1,205
18
12 to 19
915
14
946
15
20 to 24
2,448
37
2,449
37
25 to 29
1,399
21
1,367
21
≥ 30
606
9
577
9
Race
White
6,333
96
6,323
96
Black
109
2
103
2
Other
128
2
118
2
Age at menarche
≥ 14
1,243
19
1,170
18
12 to 13
3,610
55
3,610
55
≤ 11
1,717
26
1,764
27
# of first degree relatives with breast cancer
0
1,584
24
1,525
23
1
3,714
57
3,744
57
2+
1,272
19
1,275
20
Prior hysterectomy
No
4,173
63.5
4,018
62.4
Yes
2,397
36.5
2,464
37.7
# of previous breast biopsies
0
2,935
45
2,923
45
1
1,833
28
1,850
28
≥ 2
1,802
27
1,771
27
History of atypical hyperplasia in the breast
No
5,958
91
5,969
91
Yes
612
9
575
9
History of LCIS at entry
No
6,165
94
6,135
94
Yes
405
6
409
6
5 year predicted breast cancer risk (%)
≤ 2.00
1,646
25
1,626
25
2.01 to 3.00
2,028
31
2,057
31
3.01 to 5.00
1,787
27
1,707
26
≥ 5.01
1,109
17
1,162
18
Total
6,570
100.0
6,544
100.0
Results are shown in Table 3. After a median follow-up of 4.2 years, the incidence of invasive breast cancer was reduced by 44% among women assigned to tamoxifen (86 cases-tamoxifen, 156 cases-placebo; p < 0.00001; relative risk (RR) = 0.56, 95% CI: 0.43 to 0.72). A reduction in the incidence of breast cancer was seen in each prospectively specified age group (≤ 49, 50 to 59, ≥ 60), in women with or without LCIS, and in each of the absolute risk levels specified in Table 3. A non-significant decrease in the incidence of ductal carcinoma in situ (DCIS) was seen (23 tamoxifen, 35 placebo; RR = 0.66, 95% CI: 0.39 to 1.11).
There was no statistically significant difference in the number of myocardial infarctions, severe angina, or acute ischemic cardiac events between the two groups (61 tamoxifen, 59 placebo; RR = 1.04, 95% CI: 0.73 to 1.49).
No overall difference in mortality (53 deaths in tamoxifen group vs. 65 deaths in placebo group) was present. No difference in breast cancer-related mortality was observed (4 deaths in tamoxifen group vs. 5 deaths in placebo group).
Although there was a non-significant reduction in the number of hip fractures (9 on tamoxifen, 20 on placebo) in the tamoxifen group, the number of wrist fractures was similar in the two treatment groups (69 on tamoxifen, 74 on placebo). A subgroup analysis of the P-1 trial, suggests a difference in effect in bone mineral density (BMD) related to menopausal status in patients receiving tamoxifen. In postmenopausal women there was no evidence of bone loss of the lumbar spine and hip. Conversely, tamoxifen was associated with significant bone loss of the lumbar spine and hip in premenopausal women.
The risks of tamoxifen therapy include endometrial cancer, DVT, PE, stroke, cataract formation, and cataract surgery (see Table 3). In the NSABP P-1 trial, 33 cases of endometrial cancer were observed in the tamoxifen group vs. 14 in the placebo group (RR = 2.48, 95% CI: 1.27 to 4.92). Deep-vein thrombosis was observed in 30 women receiving tamoxifen vs. 19 in women receiving placebo (RR = 1.59, 95% CI: 0.86 to 2.98). Eighteen cases of pulmonary embolism were observed in the tamoxifen group vs. 6 in the placebo group (RR = 3.01, 95% CI: 1.15 to 9.27). There were 34 strokes on the tamoxifen arm and 24 on the placebo arm (RR = 1.42, 95% CI: 0.82 to 2.51). Cataract formation in women without cataracts at baseline was observed in 540 women taking tamoxifen vs. 483 women receiving placebo (RR = 1.13, 95% CI: 1.00 to 1.28). Cataract surgery (with or without cataracts at baseline) was performed in 201 women taking tamoxifen vs. 129 women receiving placebo (RR = 1.51, 95% CI: 1.21 to 1.89) (see WARNINGS).
Table 3 summarizes the major outcomes of the NSABP P-1 trial. For each endpoint, the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits or risks of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.
For most participants, multiple risk factors would have been required for eligibility. This table considers risk factors individually, regardless of other co-existing risk factors, for women who developed breast cancer. The 5 year predicted absolute breast cancer risk accounts for multiple risk factors in an individual and should provide the best estimate of individual benefit (see INDICATIONS AND USAGE).
Table 3. Major Outcomes of the NSABP P-1 Trial - * Two women had hip and wrist fractures
- † Includes Colles' and other lower radius fractures
- ‡ Requiring angioplasty or CABG
- § New Q-wave on ECG; no angina or elevation of serum enzymes; or angina requiring hospitalization without surgery
- ¶ Updated long-term follow-up data (median 6.9 years) from NSABP P-1 study added after cut-off for the other information in this table.
- # Seven cases were fatal; three in the placebo group and four in the tamoxifen group
- Þ Three cases in the tamoxifen group were fatal
- ß All but three cases in each group required hospitalization
- à Based on women without cataracts at baseline (6,230 Placebo, 6,199 Tamoxifen)
- è All women (6,707 Placebo, 6,681 Tamoxifen)
TYPE OF EVENT
# OF EVENTS
RATE/1,000 WOMEN/YEAR
95% CI
PLACEBO
TAMOXIFEN
PLACEBO
TAMOXIFEN
RR
LIMITS
Invasive Breast Cancer
156
86
6.49
3.58
0.56
0.43 to 0.72
Age ≤ 49
59
38
6.34
4.11
0.65
0.43 to 0.98
Age 50 to 59
46
25
6.31
3.53
0.56
0.35 to 0.91
Age ≥ 60
51
23
7.17
3.22
0.45
0.27 to 0.74
Risk Factors for Breast Cancer History, LCIS
No
140
78
6.23
3.51
0.56
0.43 to 0.74
Yes
16
8
12.73
6.33
0.50
0.21 to 1.17
History, Atypical Hyperplasia
No
138
84
6.37
3.89
0.61
0.47 to 0.80
Yes
18
2
8.69
1.05
0.12
0.03 to 0.52
No. First Degree Relatives
0
32
17
5.97
3.26
0.55
0.30 to 0.98
1
80
45
5.81
3.31
0.57
0.40 to 0.82
2
35
18
8.92
4.67
0.52
0.30 to 0.92
≥ 3
9
6
13.33
7.58
0.57
0.20 to 1.59
5 Year Predicted Breast Cancer Risk (as calculated by the Gail Model)
≤ 2.00%
31
13
5.36
2.26
0.42
0.22 to 0.81
2.01 to 3.00%
39
28
5.25
3.83
0.73
0.45 to 1.18
3.01 to 5.00%
36
26
5.37
4.06
0.76
0.46 to 1.26
≥ 5.00%
50
19
13.15
4.71
0.36
0.21 to 0.61
DCIS
35
23
1.47
0.97
0.66
0.39 to 1.11
Fractures (protocol-specified sites)
92*
76*
3.87
3.20
0.61
0.83 to 1.12
Hip
20
9
0.84
0.38
0.45
0.18 to 1.04
Wrist†
74
69
3.11
2.91
0.93
0.67 to 1.29
Total Ischemic Events
59
61
2.47
2.57
1.04
0.71 to 1.51
Myocardial Infarction
27
27
1.13
1.13
1.00
0.57 to 1.78
Fatal
8
7
0.33
0.29
0.88
0.27 to 2.77
Nonfatal
19
20
0.79
0.84
1.06
0.54 to 2.09
Angina‡
12
12
0.50
0.50
1.00
0.41 to 2.44
Acute Ischemic Syndrome§
20
22
0.84
0.92
1.11
0.58 to 2.13
Uterine Malignancies (among women with an intact uterus)¶
17
57
Endometrial Adenocarcinoma¶
17
53
0.71
2.20
Uterine Sarcoma¶
0
4
0.0
0.17
Stroke#
24
34
1.00
1.43
1.42
0.82 to 2.51
Transient Ischemic Attack
21
18
0.88
0.75
0.86
0.43 to 1.70
Pulmonary EmboliÞ
6
18
0.25
0.75
3.01
1.15 to 9.27
Deep-Vein Thrombosisß
19
30
0.79
1.26
1.59
0.86 to 2.98
Cataracts Developing on Studyà
483
540
22.51
25.41
1.13
1.00 to 1.28
Underwent Cataract Surgeryà
63
101
2.83
4.57
1.62
1.18 to 2.22
Underwent Cataract Surgeryè
129
201
5.44
8.56
1.58
1.26 to 1.97
Table 4 describes the characteristics of the breast cancers in the NSABP P-1 trial and includes tumor size, nodal status, ER status. Tamoxifen decreased the incidence of small estrogen receptor positive tumors, but did not alter the incidence of estrogen receptor negative tumors or larger tumors.
Table 4: Characteristics of Breast Cancer in NSABP P-1 Trial - * One participant presented with a suspicious bone scan but did not have documented metastases. She subsequently died of metastatic breast cancer.
Staging Parameter
Placebo
Tamoxifen
Total
N = 156
N = 86
N = 242
Tumor size:
T1
117
60
177
T2
28
20
48
T3
7
3
10
T4
1
2
3
Unknown
3
1
4
Nodal status:
Negative
103
56
159
1 to 3 positive nodes
29
14
43
≥ 4 positive nodes
10
12
22
Unknown
14
4
18
Stage:
I
88
47
135
II: node negative
15
9
24
II: node positive
33
22
55
III
6
4
10
IV
2*
1
3
Unknown
12
3
15
Estrogen receptor:
Positive
115
38
153
Negative
27
36
63
Unknown
14
12
26
Interim results from 2 trials in addition to the NSABP P-1 trial examining the effects of tamoxifen in reducing breast cancer incidence have been reported.
The first was the Italian Tamoxifen Prevention trial. In this trial women between the ages of 35 and 70, who had had a total hysterectomy, were randomized to receive 20 mg tamoxifen or matching placebo for 5 years. The primary endpoints were occurrence of, and death from, invasive breast cancer. Women without any specific risk factors for breast cancer were to be entered. Between 1992 and 1997, 5,408 women were randomized. Hormone Replacement Therapy (HRT) was used in 14% of participants. The trial closed in 1997 due to the large number of dropouts during the first year of treatment (26%). After 46 months of follow-up there were 22 breast cancers in women on placebo and 19 in women on tamoxifen. Although no decrease in breast cancer incidence was observed, there was a trend for reduction in breast cancer among women receiving protocol therapy for at least 1 year (19 placebo, 11 tamoxifen). The small numbers of participants along with the low level of risk in this otherwise healthy group precluded an adequate assessment of the effect of tamoxifen in reducing the incidence of breast cancer.
The second trial, the Royal Marsden Trial (RMT) was reported as an interim analysis. The RMT was begun in 1986 as a feasibility study of whether larger scale trials could be mounted. The trial was subsequently extended to a pilot trial to accrue additional participants to further assess the safety of tamoxifen. Twenty-four hundred and seventy-one women were entered between 1986 and 1996; they were selected on the basis of a family history of breast cancer. HRT was used in 40% of participants. In this trial, with a 70 month median follow-up, 34 and 36 breast cancers (8 noninvasive, 4 on each arm) were observed among women on tamoxifen and placebo, respectively. Patients in this trial were younger than those in the NSABP P-1 trial and may have been more likely to develop ER (-) tumors, which are unlikely to be reduced in number by tamoxifen therapy. Although women were selected on the basis of family history and were thought to have a high risk of breast cancer, few events occurred, reducing the statistical power of the study. These factors are potential reasons why the RMT may not have provided an adequate assessment of the effectiveness of tamoxifen in reducing the incidence of breast cancer.
In these trials, an increased number of cases of deep-vein thrombosis, pulmonary embolus, stroke, and endometrial cancer were observed on the tamoxifen arm compared to the placebo arm. The frequency of events was consistent with the safety data observed in the NSABP P-1 trial.
McCune-Albright Syndrome
A single, uncontrolled multicenter trial of tamoxifen 20 mg once a day was conducted in a heterogenous group of girls with McCune-Albright syndrome and precocious puberty manifested by physical signs of pubertal development, episodes of vaginal bleeding and/or advanced bone age (bone age of at least 12 months beyond chronological age). Twenty-eight female pediatric patients, aged 2 to 10 years, were treated for up to 12 months. Effect of treatment on frequency of vaginal bleeding, bone age advancement, and linear growth rate was assessed relative to prestudy baseline. Tamoxifen treatment was associated with a 50% reduction in frequency of vaginal bleeding episodes by patient or family report (mean annualized frequency of 3.56 episodes at baseline and 1.73 episodes on-treatment). Among the patients who reported vaginal bleeding during the prestudy period, 62% (13 out of 21 patients) reported no bleeding for a 6 month period and 33% (7 out of 21 patients) reported no vaginal bleeding for the duration of the trial. Not all patients improved on treatment and a few patients not reporting vaginal bleeding in the 6 months prior to enrollment reported menses on treatment. Tamoxifen therapy was associated with a reduction in mean rate of increase of bone age. Individual responses with regard to bone age advancement were highly heterogeneous. Linear growth rate was reduced during the course of tamoxifen treatment in a majority of patients (mean change of 1.68 cm/year relative to baseline; change from 7.47 cm/year at baseline to 5.79 cm/year on study). This change was not uniformly seen across all stages of bone maturity; all recorded response failures occurred in patients with bone ages less than 7 years at screening.
Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted in adults treated with tamoxifen (see BOXED WARNING), continued monitoring of McCune-Albright patients treated with tamoxifen for long-term uterine effects is recommended. The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy in girls have not been established.
-
INDICATIONS AND USAGE
Metastatic Breast Cancer
Tamoxifen citrate tablets are effective in the treatment of metastatic breast cancer in women and men. In premenopausal women with metastatic breast cancer, tamoxifen is an alternative to oophorectomy or ovarian irradiation. Available evidence indicates that patients whose tumors are estrogen receptor positive are more likely to benefit from tamoxifen therapy.
Adjuvant Treatment of Breast Cancer
Tamoxifen citrate tablets are indicated for the treatment of node-positive breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation. In some tamoxifen adjuvant studies, most of the benefit to date has been in the subgroup with four or more positive axillary nodes.
Tamoxifen citrate tablets are indicated for the treatment of axillary node-negative breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation.
The estrogen and progesterone receptor values may help to predict whether adjuvant tamoxifen therapy is likely to be beneficial.
Tamoxifen reduces the occurrence of contralateral breast cancer in patients receiving adjuvant tamoxifen therapy for breast cancer.
Ductal Carcinoma in Situ (DCIS)
In women with DCIS, following breast surgery and radiation, tamoxifen citrate tablets are indicated to reduce the risk of invasive breast cancer (see BOXED WARNING at the beginning of the label). The decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy.
Current data from clinical trials support 5 years of adjuvant tamoxifen therapy for patients with breast cancer.
Reduction in Breast Cancer Incidence in High Risk Women
Tamoxifen citrate tablets are indicated to reduce the incidence of breast cancer in women at high risk for breast cancer. This effect was shown in a study of 5 years planned duration with a median follow-up of 4.2 years. Twenty-five percent of the participants received drug for 5 years. The longer-term effects are not known. In this study, there was no impact of tamoxifen on overall or breast cancer-related mortality (see BOXED WARNING at the beginning of the label).
Tamoxifen citrate tablets are indicated only for high-risk women. "High risk" is defined as women at least 35 years of age with a 5 year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
Examples of combinations of factors predicting a 5 year risk ≥ 1.67% are:
Age 35 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer, 2 or more benign biopsies, and a history of a breast biopsy showing atypical hyperplasia; or
- At least 2 first degree relatives with a history of breast cancer, and a personal history of at least 1 breast biopsy; or
- LCIS
Age 40 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer, 2 or more benign biopsies, age at first live birth 25 or older, and age at menarche 11 or younger; or
- At least 2 first degree relatives with a history of breast cancer, and age at first live birth 19 or younger; or
- One first degree relative with a history of breast cancer, and a personal history of a breast biopsy showing atypical hyperplasia.
Age 45 or older and any of the following combination of factors:
- At least 2 first degree relatives with a history of breast cancer and age at first live birth 24 or younger; or
- One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, age at menarche 11 or less and age at first live birth 20 or more.
Age 50 or older and any of the following combination of factors:
- At least 2 first degree relatives with a history of breast cancer; or
- History of 1 breast biopsy showing atypical hyperplasia, and age at first live birth 30 or older and age at menarche 11 or less; or
- History of at least 2 breast biopsies with a history of atypical hyperplasia, and age at first live birth 30 or more.
Age 55 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, and age at menarche 11 or less; or
- History of at least 2 breast biopsies with a history of atypical hyperplasia, and age at first live birth 20 or older.
Age 60 or older and:
- Five-year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
For women whose risk factors are not described in the above examples, the Gail Model is necessary to estimate absolute breast cancer risk. Health Care Professionals can obtain a Gail Model Risk Assessment Tool by dialing 1-844-825-8500.
There are insufficient data available regarding the effect of tamoxifen on breast cancer incidence in women with inherited mutations (BRCA1, BRCA2) to be able to make specific recommendations on the effectiveness of tamoxifen in these patients.
After an assessment of the risk of developing breast cancer, the decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy. In the NSABP P-1 trial, tamoxifen treatment lowered the risk of developing breast cancer during the follow-up period of the trial, but did not eliminate breast cancer risk (see Table 3 in CLINICAL PHARMACOLOGY).
- CONTRAINDICATIONS
-
WARNINGS
Effects in Metastatic Breast Cancer Patients
As with other additive hormonal therapy (estrogens and androgens), hypercalcemia has been reported in some breast cancer patients with bone metastases within a few weeks of starting treatment with tamoxifen. If hypercalcemia does occur, appropriate measures should be taken and, if severe, tamoxifen should be discontinued.
Effects on the Uterus-Endometrial Cancer and Uterine Sarcoma
An increased incidence of uterine malignancies has been reported in association with tamoxifen treatment. The underlying mechanism is unknown, but may be related to the estrogen-like effect of tamoxifen. Most uterine malignancies seen in association with tamoxifen are classified as adenocarcinoma of the endometrium. However, rare uterine sarcomas, including malignant mixed mullerian tumors (MMMT), have also been reported. Uterine sarcoma is generally associated with a higher FIGO stage (III/IV) at diagnosis, poorer prognosis, and shorter survival. Uterine sarcoma has been reported to occur more frequently among long-term users (≥ 2 years) of tamoxifen than non-users. Some of the uterine malignancies (endometrial carcinoma or uterine sarcoma) have been fatal.
In the NSABP P-1 trial, among participants randomized to tamoxifen there was a statistically significant increase in the incidence of endometrial cancer (33 cases of invasive endometrial cancer, compared to 14 cases among participants randomized to placebo (RR = 2.48, 95% CI: 1.27 to 4.92). The 33 cases in participants receiving tamoxifen were FIGO Stage I, including 20 IA, 12 IB, and 1 IC endometrial adenocarcinomas. In participants randomized to placebo, 13 were FIGO Stage I (8 IA and 5 IB) and 1 was FIGO Stage IV. Five women on tamoxifen and 1 on placebo received postoperative radiation therapy in addition to surgery. This increase was primarily observed among women at least 50 years of age at the time of randomization (26 cases of invasive endometrial cancer, compared to 6 cases among participants randomized to placebo (RR = 4.50, 95% CI: 1.78 to 13.16). Among women ≤ 49 years of age at the time of randomization there were 7 cases of invasive endometrial cancer, compared to 8 cases among participants randomized to placebo (RR = 0.94, 95% CI: 0.28 to 2.89). If age at the time of diagnosis is considered, there were 4 cases of endometrial cancer among participants ≤ 49 randomized to tamoxifen compared to 2 among participants randomized to placebo (RR = 2.21, 95% CI: 0.4 to 12.0). For women ≥ 50 at the time of diagnosis, there were 29 cases among participants randomized to tamoxifen compared to 12 among women on placebo (RR = 2.5, 95% CI: 1.3 to 4.9). The risk ratios were similar in the two groups, although fewer events occurred in younger women. Most (29 of 33 cases in the tamoxifen group) endometrial cancers were diagnosed in symptomatic women, although 5 of 33 cases in the tamoxifen group occurred in asymptomatic women. Among women receiving tamoxifen the events appeared between 1 and 61 months (average = 32 months) from the start of treatment.
In an updated review of long-term data (median length of total follow-up is 6.9 years, including blinded follow-up) on 8,306 women with an intact uterus at randomization in the NSABP P-1 risk reduction trial, the incidence of both adenocarcinomas and rare uterine sarcomas was increased in women taking tamoxifen. During blinded follow-up, there were 36 cases of FIGO Stage I endometrial adenocarcinoma (22 were FIGO Stage IA, 13 IB, and 1 IC) in women receiving tamoxifen and 15 cases in women receiving placebo [14 were FIGO Stage I (9 IA and 5 IB), and 1 case was FIGO Stage IV]. Of the patients receiving tamoxifen who developed endometrial cancer, one with Stage IA and 4 with Stage IB cancers received radiation therapy. In the placebo group, one patient with FIGO Stage IB cancer received radiation therapy and the patient with FIGO Stage IVB cancer received chemotherapy and hormonal therapy. During total follow-up, endometrial adenocarcinoma was reported in 53 women randomized to tamoxifen (30 cases of FIGO Stage IA, 20 were Stage IB, 1 was Stage IC, and 2 were Stage IIIC), and 17 women randomized to placebo (9 cases were FIGO Stage IA, 6 were Stage IB, 1 was Stage IIIC, and 1 was Stage IVB) (incidence per 1,000 women-years of 2.20 and 0.71, respectively). Some patients received postoperative radiation therapy in addition to surgery. Uterine sarcomas were reported in 4 women randomized to tamoxifen (1 was FIGO IA, 1 was FIGO IB, 1 was FIGO IIA, and 1 was FIGO IIIC) and 1 patient randomized to placebo (FIGO 1A); incidence per 1,000 women-years of 0.17 and 0.04, respectively. Of the patients randomized to tamoxifen, the FIGO IA and IB cases were a MMMT and sarcoma, respectively; the FIGO II was a MMMT; and the FIGO III was a sarcoma; and the 1 patient randomized to placebo had a MMMT. A similar increased incidence in endometrial adenocarcinoma and uterine sarcoma was observed among women receiving tamoxifen in 5 other NSABP clinical trials.
Any patient receiving or who has previously received tamoxifen who reports abnormal vaginal bleeding should be promptly evaluated. Patients receiving or who have previously received tamoxifen should have annual gynecological examinations and they should promptly inform their physicians if they experience any abnormal gynecological symptoms, e.g., menstrual irregularities, abnormal vaginal bleeding, changes in vaginal discharge, or pelvic pain or pressure.
In the P-1 trial, endometrial sampling did not alter the endometrial cancer detection rate compared to women who did not undergo endometrial sampling (0.6% with sampling, 0.5% without sampling) for women with an intact uterus. There are no data to suggest that routine endometrial sampling in asymptomatic women taking tamoxifen to reduce the incidence of breast cancer would be beneficial.
Non-Malignant Effects on the Uterus
An increased incidence of endometrial changes including hyperplasia and polyps has been reported in association with tamoxifen treatment. The incidence and pattern of this increase suggest that the underlying mechanism is related to the estrogenic properties of tamoxifen.
There have been a few reports of endometriosis and uterine fibroids in women receiving tamoxifen. The underlying mechanism may be due to the partial estrogenic effect of tamoxifen. Ovarian cysts have also been observed in a small number of premenopausal patients with advanced breast cancer who have been treated with tamoxifen.
Tamoxifen has been reported to cause menstrual irregularity or amenorrhea.
Thromboembolic Effects of Tamoxifen
There is evidence of an increased incidence of thromboembolic events, including deep-vein thrombosis and pulmonary embolism, during tamoxifen therapy. When tamoxifen is coadministered with chemotherapy, there may be a further increase in the incidence of thromboembolic effects. For treatment of breast cancer, the risks and benefits of tamoxifen should be carefully considered in women with a history of thromboembolic events. In a small substudy (N = 81) of the NSABP-1 trial, there appeared to be no benefit to screening women for Factor V Leiden and Prothrombin mutations G20210A as a means to identify those who may not be appropriate candidates for tamoxifen therapy.
Data from the NSABP P-1 trial show that participants receiving tamoxifen without a history of pulmonary emboli (PE) had a statistically significant increase in pulmonary emboli (18 tamoxifen, 6 placebo; RR = 3.01, 95% CI: 1.15 to 9.27). Three of the pulmonary emboli, all in the tamoxifen arm, were fatal. Eighty-seven percent of the cases of pulmonary embolism occurred in women at least 50 years of age at randomization. Among women receiving tamoxifen, the events appeared between 2 and 60 months (average = 27 months) from the start of treatment.
In this same population, a non-statistically significant increase in deep-vein thrombosis (DVT) was seen in the tamoxifen group (30-tamoxifen, 19-placebo; RR = 1.59, 95% CI: 0.86 to 2.98). The same increase in relative risk was seen in women ≤ 49 and in women ≥ 50, although fewer events occurred in younger women. Women with thromboembolic events were at risk for a second related event (7 out of 25 women on placebo, 5 out of 48 women on tamoxifen) and were at risk for complications of the event and its treatment (0/25 on placebo, 4/48 on tamoxifen). Among women receiving tamoxifen, deep-vein thrombosis events occurred between 2 and 57 months (average = 19 months) from the start of treatment.
There was a non-statistically significant increase in stroke among patients randomized to tamoxifen (24 placebo; 34 tamoxifen; RR = 1.42, 95% CI: 0.82 to 2.51). Six of the 24 strokes in the placebo group were considered hemorrhagic in origin and 10 of the 34 strokes in the tamoxifen group were categorized as hemorrhagic. Seventeen of the 34 strokes in the tamoxifen group were considered occlusive and 7 were considered to be of unknown etiology. Fourteen of the 24 strokes on the placebo arm were reported to be occlusive and 4 of unknown etiology. Among these strokes 3 strokes in the placebo group and 4 strokes in the tamoxifen group were fatal. Eighty-eight percent of the strokes occurred in women at least 50 years of age at the time of randomization. Among women receiving tamoxifen, the events occurred between 1 and 63 months (average = 30 months) from the start of treatment.
Effects on the Liver: Liver Cancer
In the Swedish trial using adjuvant tamoxifen 40 mg/day for 2 to 5 years, 3 cases of liver cancer have been reported in the tamoxifen-treated group vs. 1 case in the observation group (see PRECAUTIONS, Carcinogenesis). In other clinical trials evaluating tamoxifen, no cases of liver cancer have been reported to date.
One case of liver cancer was reported in NSABP P-1 in a participant randomized to tamoxifen.
Effects on the Liver: Non-Malignant Effects
Tamoxifen has been associated with changes in liver enzyme levels, and on rare occasions, a spectrum of more severe liver abnormalities including fatty liver, cholestasis, hepatitis and hepatic necrosis. A few of these serious cases included fatalities. In most reported cases the relationship to tamoxifen is uncertain. However, some positive rechallenges and dechallenges have been reported.
In the NSABP P-1 trial, few grade 3 to 4 changes in liver function (SGOT, SGPT, bilirubin, alkaline phosphatase) were observed (10 on placebo and 6 on tamoxifen). Serum lipids were not systematically collected.
Other Cancers
A number of second primary tumors, occurring at sites other than the endometrium, have been reported following the treatment of breast cancer with tamoxifen in clinical trials. Data from the NSABP B-14 and P-1 studies show no increase in other (non-uterine) cancers among patients receiving tamoxifen. Whether an increased risk for other (non-uterine) cancers is associated with tamoxifen is still uncertain and continues to be evaluated.
Effects on the Eye
Ocular disturbances, including corneal changes, decrement in color vision perception, retinal vein thrombosis, and retinopathy have been reported in patients receiving tamoxifen. An increased incidence of cataracts and the need for cataract surgery have been reported in patients receiving tamoxifen.
In the NSABP P-1 trial, an increased risk of borderline significance of developing cataracts among those women without cataracts at baseline (540 tamoxifen; 483 placebo; RR = 1.13, 95% CI: 1.00 to 1.28) was observed. Among these same women, tamoxifen was associated with an increased risk of having cataract surgery (101 tamoxifen; 63 placebo; RR = 1.62, 95% CI: 1.18 to 2.22) (see Table 3 in CLINICAL PHARMACOLOGY). Among all women on the trial (with or without cataracts at baseline), tamoxifen was associated with an increased risk of having cataract surgery (201 tamoxifen; 129 placebo; RR = 1.58, 95% CI: 1.26 to 1.97). Eye examinations were not required during the study. No other conclusions regarding non-cataract ophthalmic events can be made.
Pregnancy Category D
Tamoxifen may cause fetal harm when administered to a pregnant woman. Women should be advised not to become pregnant while taking tamoxifen or within 2 months of discontinuing tamoxifen and should use barrier or nonhormonal contraceptive measures if sexually active. Tamoxifen does not cause infertility, even in the presence of menstrual irregularity. Effects on reproductive functions are expected from the antiestrogenic properties of the drug. In reproductive studies in rats at dose levels equal to or below the human dose, nonteratogenic developmental skeletal changes were seen and were found reversible. In addition, in fertility studies in rats and in teratology studies in rabbits using doses at or below those used in humans, a lower incidence of embryo implantation and a higher incidence of fetal death or retarded in utero growth were observed, with slower learning behavior in some rat pups when compared to historical controls. Several pregnant marmosets were dosed with 10 mg/kg/day (about 2 fold the daily maximum recommended human dose on a mg/m2 basis) during organogenesis or in the last half of pregnancy. No deformations were seen and, although the dose was high enough to terminate pregnancy in some animals, those that did maintain pregnancy showed no evidence of teratogenic malformations.
In rodent models of fetal reproductive tract development, tamoxifen (at doses 0.002 to 2.4 fold the daily maximum recommended human dose on a mg/m2 basis) caused changes in both sexes that are similar to those caused by estradiol, ethynylestradiol and diethylstilbestrol. Although the clinical relevance of these changes is unknown, some of these changes, especially vaginal adenosis, are similar to those seen in young women who were exposed to diethylstilbestrol in utero and who have a 1 in 1,000 risk of developing clear-cell adenocarcinoma of the vagina or cervix. To date, in utero exposure to tamoxifen has not been shown to cause vaginal adenosis, or clear-cell adenocarcinoma of the vagina or cervix, in young women. However, only a small number of young women have been exposed to tamoxifen in utero, and a smaller number have been followed long enough (to age 15 to 20) to determine whether vaginal or cervical neoplasia could occur as a result of this exposure.
There are no adequate and well-controlled trials of tamoxifen in pregnant women. There have been a small number of reports of vaginal bleeding, spontaneous abortions, birth defects, and fetal deaths in pregnant women. If this drug is used during pregnancy, or the patient becomes pregnant while taking this drug, or within approximately two months after discontinuing therapy, the patient should be apprised of the potential risks to the fetus including the potential long-term risk of a DES-like syndrome.
Reduction in Breast Cancer Incidence in High Risk Women
Pregnancy Category D
For sexually active women of child-bearing potential, tamoxifen therapy should be initiated during menstruation. In women with menstrual irregularity, a negative B-HCG immediately prior to the initiation of therapy is sufficient (see PRECAUTIONS, Information for Patients, Reduction in Breast Cancer Incidence in High Risk Women).
-
PRECAUTIONS
General
Decreases in platelet counts, usually to 50,000 to 100,000/mm3, infrequently lower, have been occasionally reported in patients taking tamoxifen for breast cancer. In patients with significant thrombocytopenia, rare hemorrhagic episodes have occurred, but it is uncertain if these episodes are due to tamoxifen therapy. Leukopenia has been observed, sometimes in association with anemia and/or thrombocytopenia. There have been rare reports of neutropenia and pancytopenia in patients receiving tamoxifen; this can sometimes be severe.
In the NSABP P-1 trial, 6 women on tamoxifen and 2 on placebo experienced grade 3 to 4 drops in platelet counts (≤ 50,000/mm3).
Information for Patients
Patients should be instructed to read the Medication Guide supplied as required by law when tamoxifen is dispensed. The complete text of the Medication Guide is reprinted at the end of this document.
Reduction in Invasive Breast Cancer and DCIS in Women With DCIS
Women with DCIS treated with lumpectomy and radiation therapy who are considering tamoxifen to reduce the incidence of a second breast cancer event should assess the risks and benefits of therapy, since treatment with tamoxifen decreased the incidence of invasive breast cancer, but has not been shown to affect survival (see Table 1 in CLINICAL PHARMACOLOGY).
Reduction in Breast Cancer Incidence in High Risk Women
Women who are at high risk for breast cancer can consider taking tamoxifen therapy to reduce the incidence of breast cancer. Whether the benefits of treatment are considered to outweigh the risks depends on a woman's personal health history and on how she weighs the benefits and risks. Tamoxifen therapy to reduce the incidence of breast cancer may therefore not be appropriate for all women at high risk for breast cancer. Women who are considering tamoxifen therapy should consult their health care professional for an assessment of the potential benefits and risks prior to starting therapy for reduction in breast cancer incidence (see Table 3 in CLINICAL PHARMACOLOGY). Women should understand that tamoxifen reduces the incidence of breast cancer, but may not eliminate risk. Tamoxifen decreased the incidence of small estrogen receptor positive tumors, but did not alter the incidence of estrogen receptor negative tumors or larger tumors. In women with breast cancer who are at high risk of developing a second breast cancer, treatment with about 5 years of tamoxifen reduced the annual incidence rate of a second breast cancer by approximately 50%.
Women who are pregnant or who plan to become pregnant should not take tamoxifen to reduce their risk of breast cancer. Effective nonhormonal contraception must be used by all premenopausal women taking tamoxifen and for approximately two months after discontinuing therapy if they are sexually active. Tamoxifen does not cause infertility, even in the presence of menstrual irregularity. For sexually active women of child-bearing potential, tamoxifen therapy should be initiated during menstruation. In women with menstrual irregularity, a negative B-HCG immediately prior to the initiation of therapy is sufficient (see WARNINGS, Pregnancy Category D).
Two European trials of tamoxifen to reduce the risk of breast cancer were conducted and showed no difference in the number of breast cancer cases between the tamoxifen and placebo arms. These studies had trial designs that differed from that of NSABP P-1, were smaller than NSABP P-1, and enrolled women at a lower risk for breast cancer than those in P-1.
Monitoring During Tamoxifen Therapy
Women taking or having previously taken tamoxifen should be instructed to seek prompt medical attention for new breast lumps, vaginal bleeding, gynecologic symptoms (menstrual irregularities, changes in vaginal discharge, or pelvic pain or pressure), symptoms of leg swelling or tenderness, unexplained shortness of breath, or changes in vision. Women should inform all care providers, regardless of the reason for evaluation, that they take tamoxifen.
Women taking tamoxifen to reduce the incidence of breast cancer should have a breast examination, a mammogram, and a gynecologic examination prior to the initiation of therapy. These studies should be repeated at regular intervals while on therapy, in keeping with good medical practice. Women taking tamoxifen as adjuvant breast cancer therapy should follow the same monitoring procedures as for women taking tamoxifen for the reduction in the incidence of breast cancer. Women taking tamoxifen as treatment for metastatic breast cancer should review this monitoring plan with their care provider and select the appropriate modalities and schedule of evaluation.
Laboratory Tests
Periodic complete blood counts, including platelet counts, and periodic liver function tests should be obtained.
During the ATAC trial, more patients receiving anastrozole were reported to have an elevated serum cholesterol compared to patients receiving tamoxifen (9% versus 3.5%, respectively).
Drug Interactions
When tamoxifen is used in combination with coumarin-type anticoagulants, a significant increase in anticoagulant effect may occur. Where such coadministration exists, careful monitoring of the patient's prothrombin time is recommended.
In the NSABP P-1 trial, women who required coumarin-type anticoagulants for any reason were ineligible for participation in the trial (see CONTRAINDICATIONS).
There is an increased risk of thromboembolic events occurring when cytotoxic agents are used in combination with tamoxifen.
Tamoxifen reduced letrozole plasma concentrations by 37%. The effect of tamoxifen on metabolism and excretion of other antineoplastic drugs, such as cyclophosphamide and other drugs that require mixed function oxidases for activation, is not known. Tamoxifen and N-desmethyl tamoxifen plasma concentrations have been shown to be reduced when coadministered with rifampin or aminoglutethimide. Induction of CYP3A4-mediated metabolism is considered to be the mechanism by which these reductions occur; other CYP3A4 inducing agents have not been studied to confirm this effect.
One patient receiving tamoxifen with concomitant phenobarbital exhibited a steady-state serum level of tamoxifen lower than that observed for other patients (i.e., 26 ng/mL vs. mean value of 122 ng/mL). However, the clinical significance of this finding is not known. Rifampin induced the metabolism of tamoxifen and significantly reduced the plasma concentrations of tamoxifen in 10 patients. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
Concomitant bromocriptine therapy has been shown to elevate serum tamoxifen and N-desmethyl tamoxifen.
Based on clinical and pharmacokinetic results from the anastrozole adjuvant trial, tamoxifen should not be administered with anastrozole (see CLINICAL PHARMACOLOGY, Drug-Drug Interactions).
Drug/Laboratory Testing Interactions
During postmarketing surveillance, T4 elevations were reported for a few postmenopausal patients which may be explained by increases in thyroid-binding globulin. These elevations were not accompanied by clinical hyperthyroidism.
Variations in the karyopyknotic index on vaginal smears and various degrees of estrogen effect on Pap smears have been infrequently seen in postmenopausal patients given tamoxifen.
In the postmarketing experience with tamoxifen, infrequent cases of hyperlipidemias have been reported. Periodic monitoring of plasma triglycerides and cholesterol may be indicated in patients with preexisting hyperlipidemias (see ADVERSE REACTIONS, Postmarketing Experience).
Carcinogenesis
A conventional carcinogenesis study in rats at doses of 5, 20, and 35 mg/kg/day (about one, three and seven-fold the daily maximum recommended human dose on a mg/m2 basis) administered by oral gavage for up to 2 years revealed a significant increase in hepatocellular carcinoma at all doses. The incidence of these tumors was significantly greater among rats administered 20 or 35 mg/kg/day (69%) compared to those administered 5 mg/kg/day (14%). In a separate study, rats were administered tamoxifen at 45 mg/kg/day (about nine-fold the daily maximum recommended human dose on a mg/m2 basis); hepatocellular neoplasia was exhibited at 3 to 6 months.
Granulosa cell ovarian tumors and interstitial cell testicular tumors were observed in 2 separate mouse studies. The mice were administered the trans and racemic forms of tamoxifen for 13 to 15 months at doses of 5, 20, and 50 mg/kg/day (about one-half, two, and five-fold the daily recommended human dose on a mg/m2 basis).
Mutagenesis
No genotoxic potential was found in a conventional battery of in vivo and in vitro tests with pro- and eukaryotic test systems with drug metabolizing systems. However, increased levels of DNA adducts were observed by 32P post-labeling in DNA from rat liver and cultured human lymphocytes. Tamoxifen also has been found to increase levels of micronucleus formation in vitro in human lymphoblastoid cell line (MCL-5). Based on these findings, tamoxifen is genotoxic in rodent and human MCL-5 cells.
Impairment of Fertility
Tamoxifen produced impairment of fertility and conception in female rats at doses of 0.04 mg/kg/day (about 0.01 fold the daily maximum recommended human dose on a mg/m2 basis) when dosed for two weeks prior to mating through day 7 of pregnancy. At this dose, fertility and reproductive indices were markedly reduced with total fetal mortality. Fetal mortality was also increased at doses of 0.16 mg/kg/day (about 0.03 fold the daily maximum recommended human dose on a mg/m2 basis) when female rats were dosed from days 7 to 17 of pregnancy. Tamoxifen produced abortion, premature delivery and fetal death in rabbits administered doses equal to or greater than 0.125 mg/kg/day (about 0.05 fold the daily maximum recommended human dose on a mg/m2 basis). There were no teratogenic changes in either rats or rabbits.
Nursing Mothers
Tamoxifen has been reported to inhibit lactation. Two placebo-controlled studies in over 150 women have shown that tamoxifen significantly inhibits early postpartum milk production. In both studies tamoxifen was administered within 24 hours of delivery for between 5 and 18 days. The effect of tamoxifen on established milk production is not known.
There are no data that address whether tamoxifen is excreted into human milk. If excreted, there are no data regarding the effects of tamoxifen in breast milk on the breastfed infant or breastfed animals. However, direct neonatal exposure of tamoxifen to mice and rats (not via breast milk) produced 1) reproductive tract lesions in female rodents (similar to those seen in humans after intrauterine exposure to diethylstilbestrol) and 2) functional defects of the reproductive tract in male rodents such as testicular atrophy and arrest of spermatogenesis.
It is not known if tamoxifen is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from tamoxifen, women taking tamoxifen should not breast feed.
Reduction in Breast Cancer Incidence in High Risk Women With DCIS
It is not known if tamoxifen is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from tamoxifen, women taking tamoxifen should not breast feed.
Pediatric Use
The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy for girls have not been established. In adults treated with tamoxifen, an increase in incidence of uterine malignancies, stroke and pulmonary embolism has been noted (see BOXED WARNING and CLINICAL PHARMACOLOGY, Clinical Studies, McCune-Albright Syndrome).
Geriatric Use
In the NSABP P-1 trial, the percentage of women at least 65 years of age was 16%. Women at least 70 years of age accounted for 6% of the participants. A reduction in breast cancer incidence was seen among participants in each of the subsets. A total of 28 and 10 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. Across all other outcomes, the results in this subset reflect the results observed in the subset of women at least 50 years of age. No overall differences in tolerability were observed between older and younger patients (see CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women).
In the NSABP B-24 trial, the percentage of women at least 65 years of age was 23%. Women at least 70 years of age accounted for 10% of participants. A total of 14 and 12 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. This subset is too small to reach any conclusions on efficacy. Across all other endpoints, the results in this subset were comparable to those of younger women enrolled in this trial. No overall differences in tolerability were observed between older and younger patients.
-
ADVERSE REACTIONS
Adverse reactions to tamoxifen are relatively mild and rarely severe enough to require discontinuation of treatment in breast cancer patients.
Continued clinical studies have resulted in further information which better indicates the incidence of adverse reactions with tamoxifen as compared to placebo.
Metastatic Breast Cancer
Increased bone and tumor pain and, also, local disease flare have occurred, which are sometimes associated with a good tumor response. Patients with increased bone pain may require additional analgesics. Patients with soft tissue disease may have sudden increases in the size of preexisting lesions, sometimes associated with marked erythema within and surrounding the lesions and/or the development of new lesions. When they occur, the bone pain or disease flare are seen shortly after starting tamoxifen and generally subside rapidly.
In patients treated with tamoxifen for metastatic breast cancer, the most frequent adverse reaction to tamoxifen is hot flashes.
Other adverse reactions which are seen infrequently are hypercalcemia, peripheral edema, distaste for food, pruritus vulvae, depression, dizziness, lightheadedness, headache, hair thinning and/or partial hair loss, and vaginal dryness.
Premenopausal Women
The following table summarizes the incidence of adverse reactions reported at a frequency of 2% or greater from clinical trials (Ingle, Pritchard, Buchanan) which compared tamoxifen therapy to ovarian ablation in premenopausal patients with metastatic breast cancer.
- * Some women had more than one adverse reaction.
TAMOXIFEN All Effects % of Women
OVARIAN ABLATION All Effects % of Women
Adverse Reactions*
n = 104
n = 100
Flush
33
46
Amenorrhea
16
69
Altered Menses
13
5
Oligomenorrhea
9
1
Bone Pain
6
6
Menstrual Disorder
6
4
Nausea
5
4
Cough/Coughing
4
1
Edema
4
1
Fatigue
4
1
Musculoskeletal Pain
3
0
Pain
3
4
Ovarian Cyst(s)
3
2
Depression
2
2
Abdominal Cramps
1
2
Anorexia
1
2
Male Breast Cancer
Tamoxifen is well tolerated in males with breast cancer. Reports from the literature and case reports suggest that the safety profile of tamoxifen in males is similar to that seen in women. Loss of libido and impotence have resulted in discontinuation of tamoxifen therapy in male patients. Also, in oligospermic males treated with tamoxifen, LH, FSH, testosterone and estrogen levels were elevated. No significant clinical changes were reported.
Adjuvant Breast Cancer
In the NSABP B-14 study, women with axillary node-negative breast cancer were randomized to 5 years of tamoxifen 20 mg/day or placebo following primary surgery. The reported adverse effects are tabulated below (mean follow-up of approximately 6.8 years) showing adverse events more common on tamoxifen than on placebo. The incidence of hot flashes (64% vs. 48%), vaginal discharge (30% vs. 15%), and irregular menses (25% vs. 19%) were higher with tamoxifen compared with placebo. All other adverse effects occurred with similar frequency in the 2 treatment groups, with the exception of thrombotic events; a higher incidence was seen in tamoxifen-treated patients (through 5 years, 1.7% vs. 0.4%). Two of the patients treated with tamoxifen who had thrombotic events died.
NSABP B-14 Study - * Defined as a platelet count of < 100,000/mm3
% of Women
Adverse Effect
TAMOXIFEN (n = 1,422)
PLACEBO(n = 1,437)
Hot Flashes
64
48
Fluid Retention
32
30
Vaginal Discharge
30
15
Nausea
26
24
Irregular Menses
25
19
Weight Loss (> 5%)
23
18
Skin Changes
19
15
Increased SGOT
5
3
Increased Bilirubin
2
1
Increased Creatinine
2
1
Thrombocytopenia*
2
1
Thrombotic Events
Deep-Vein Thrombosis
0.8
0.2
Pulmonary Embolism
0.5
0.2
Superficial Phlebitis
0.4
0.0
In the Eastern Cooperative Oncology Group (ECOG) adjuvant breast cancer trial, tamoxifen or placebo was administered for 2 years to women following mastectomy. When compared to placebo, tamoxifen showed a significantly higher incidence of hot flashes (19% vs. 8% for placebo). The incidence of all other adverse reactions was similar in the 2 treatment groups with the exception of thrombocytopenia where the incidence for tamoxifen was 10% vs. 3% for placebo, an observation of borderline statistical significance.
In other adjuvant studies, Toronto and Tamoxifen Adjuvant Trial Organization (NATO), women received either tamoxifen or no therapy. In the Toronto study, hot flashes were observed in 29% of patients for tamoxifen vs. 1% in the untreated group. In the NATO trial, hot flashes and vaginal bleeding were reported in 2.8% and 2.0% of women, respectively, for tamoxifen vs. 0.2% for each in the untreated group.
Anastrozole Adjuvant Trial – Study of Anastrozole Compared to Tamoxifen for Adjuvant Treatment of Early Breast Cancer (see CLINICAL PHARMACOLOGY, Clinical Studies).
At a median follow-up of 33 months, the combination of anastrozole and tamoxifen did not demonstrate any efficacy benefit when compared to tamoxifen therapy given alone in all patients as well as in the hormone receptor positive subpopulation. This treatment arm was discontinued from the trial. The median duration of adjuvant treatment for safety evaluation was 59.8 months and 59.6 months for patients receiving anastrozole 1 mg and tamoxifen 20 mg, respectively.
Adverse events occurring with an incidence of at least 5% in either treatment group during treatment or within 14 days of the end of treatment are presented in the following table.
Adverse events occurring with an incidence of at least 5% in either treatment group during treatment, or within 14 days of the end of treatment - * COSTART Coding Symbols for Thesaurus of Adverse Reaction Terms.
- † A patient may have had more than 1 adverse event, including more than 1 adverse event in the same body system.
- ‡ Vaginal hemorrhage without further diagnosis.
ANASTROZOLE 1 mg (N = 3,092)
TAMOXIFEN 20 mg (N = 3,094)
Body as a whole
Asthenia
575 (19)
544 (18)
Pain
533 (17)
485 (16)
Back pain
321 (10)
309 (10)
Headache
314 (10)
249 (8)
Abdominal pain
271 (9)
276 (9)
Infection
285 (9)
276 (9)
Accidental injury
311 (10)
303 (10)
Flu syndrome
175 (6)
195 (6)
Chest pain
200 (7)
150 (5)
Neoplasm
162 (5)
144 (5)
Cyst
138 (5)
162 (5)
Cardiovascular
Vasodilatation
1,104 (36)
1,264 (41)
Hypertension
402 (13)
349 (11)
Digestive
Nausea
343 (11)
335 (11)
Constipation
249 (8)
252 (8)
Diarrhea
265 (9)
216 (7)
Dyspepsia
206 (7)
169 (6)
Gastrointestinal disorder
210 (7)
158 (5)
Hemic and lymphatic
Lymphoedema
304 (10)
341 (11)
Anemia
113 (4)
159 (5)
Metabolic and nutritional
Peripheral edema
311 (10)
343 (11)
Weight gain
285 (9)
274 (9)
Hypercholesterolemia
278 (9)
108 (3.5)
Musculoskeletal
Arthritis
512 (17)
445 (14)
Arthralgia
467 (15)
344 (11)
Osteoporosis
325 (11)
226 (7)
Fracture
315 (10)
209 (7)
Bone pain
201 (7)
185 (6)
Arthrosis
207 (7)
156 (5)
Joint disorder
184 (6)
160 (5)
Myalgia
179 (6)
160 (5)
Nervous system
Depression
413 (13)
382 (12)
Insomnia
309 (10)
281 (9)
Dizziness
236 (8)
234 (8)
Anxiety
195 (6)
180 (6)
Paraesthesia
215 (7)
145 (5)
Respiratory
Pharyngitis
443 (14)
422 (14)
Cough increased
261 (8)
287 (9)
Dyspnea
234 (8)
237 (8)
Sinusitis
184 (6)
159 (5)
Bronchitis
167 (5)
153 (5)
Skin and appendages
Rash
333 (11)
387 (13)
Sweating
145 (5)
177 (6)
Special Senses
Cataract specified
182 (6)
213 (7)
Urogenital
Leukorrhea
86 (3)
286 (9)
Urinary tract infection
244 (8)
313 (10)
Breast pain
251 (8)
169 (6)
Breast neoplasm
164 (5)
139 (5)
Vulvovaginitis
194 (6)
150 (5)
Vaginal hemorrhage‡
122 (4)
180 (6)
Vaginitis
125 (4)
158 (5)
N = Number of patients receiving the treatment.
** The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up.
Certain adverse events and combinations of adverse events were prospectively specified for analysis, based on the known pharmacologic properties and side effect profiles of the two drugs (see the following table).
Number (%) of Patients with Pre-Specified Adverse Event in the Anastrozole Adjuvant Trial* - * Patients with multiple events in the same category are counted only once in that category.
- † The odds ratios < 1.00 favor anastrozole and those > 1.00 favor tamoxifen.
- ‡ Refers to joint symptoms, including joint disorder, arthritis, arthrosis and arthralgia.
- § Percentages calculated based upon the numbers of patients with an intact uterus at baseline.
Anastrozole N = 3,092 (%)
Tamoxifen N = 3,094 (%)
Odds- Ratio†
95% CI†
Hot Flashes
1,104 (36)
1,264 (41)
0.80
0.73 to 0.89
Musculoskeletal Events‡
1,100 (36)
911 (29)
1.32
1.19 to 1.47
Fatigue/Asthenia
575 (19)
544 (18)
1.07
0.94 to 1.22
Mood Disturbances
597 (19)
554 (18)
1.10
0.97 to 1.25
Nausea and Vomiting
393 (13)
384 (12)
1.03
0.88 to 1.19
All Fractures
315 (10)
209 (7)
1.57
1.30 to 1.88
Fractures of Spine, Hip, or Wrist
133 (4)
91 (3)
1.48
1.13 to 1.95
Wrist/Colles' fractures
67 (2)
50 (2)
Spine fractures
43 (1)
22 (1)
Hip fractures
28 (1)
26 (1)
Cataracts
182 (6)
213 (7)
0.85
0.69 to 1.04
Vaginal Bleeding
167 (5)
317 (10)
0.50
0.41 to 0.61
Ischemic Cardiovascular Disease
127 (4)
104 (3)
1.23
0.95 to 1.60
Vaginal Discharge
109 (4)
408 (13)
0.24
0.19 to 0.30
Venous Thromboembolic Events
87 (3)
140 (5)
0.61
0.47 to 0.80
Deep Venous Thromboembolic Events
48 (2)
74 (2)
0.64
0.45 to 0.93
Ischemic Cerebrovascular Event
62 (2)
88 (3)
0.70
0.50 to 0.97
Endometrial Cancer§
4 (0.2)
13 (0.6)
0.31
0.10 to 0.94
Patients receiving anastrozole had an increase in joint disorders (including arthritis, arthrosis and arthralgia) compared with patients receiving tamoxifen. Patients receiving anastrozole had an increase in the incidence of all fractures (specifically fractures of spine, hip and wrist) [315 (10%)] compared with patients receiving tamoxifen [209 (7%)]. Patients receiving anastrozole had a decrease in hot flashes, vaginal bleeding, vaginal discharge, endometrial cancer, venous thromboembolic events and ischemic cerebrovascular events compared with patients receiving tamoxifen.
Patients receiving tamoxifen had a decrease in hypercholesterolemia [108 (3.5%)] compared to patients receiving anastrozole [278 (9%)]. Angina pectoris was reported in 71 (2.3%) patients in the anastrozole arm and 51 (1.6%) patients in the tamoxifen arm; myocardial infarction was reported in 37 (1.2%) patients in the anastrozole arm and in 34 (1.1%) patients in the tamoxifen arm.
Results from the adjuvant trial bone substudy, at 12 and 24 months demonstrated that patients receiving anastozole had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline.
Ductal Carcinoma in Situ (DCIS)
The type and frequency of adverse events in the NSABP B-24 trial were consistent with those observed in the other adjuvant trials conducted with tamoxifen.
Reduction in Breast Cancer Incidence in High Risk Women
In the NSABP P-1 trial, there was an increase in five serious adverse effects in the tamoxifen group: endometrial cancer (33 cases in the tamoxifen group vs. 14 in the placebo group); pulmonary embolism (18 cases in the tamoxifen group vs. 6 in the placebo group); deep-vein thrombosis (30 cases in the tamoxifen group vs. 19 in the placebo group); stroke (34 cases in the tamoxifen group vs. 24 in the placebo group); cataract formation (540 cases in the tamoxifen group vs. 483 in the placebo group) and cataract surgery (101 cases in the tamoxifen group vs. 63 in the placebo group) (see WARNINGS and Table 3 in CLINICAL PHARMACOLOGY).
The following table presents the adverse events observed in NSABP P-1 by treatment arm. Only adverse events more common on tamoxifen than placebo are shown.
NSABP P-1 Trial: All Adverse Events - * Number with Quality of Life Questionnaires
- † Number with Treatment Follow-up Forms
- ‡ Number with Adverse Drug Reaction Forms
% of Women
TAMOXIFEN
PLACEBO
N = 6,681
N = 6,707
Self Reported Symptoms
N = 6,441*
N = 6,469*
Hot Flashes
80
68
Vaginal Discharges
55
35
Vaginal Bleeding
23
22
Laboratory Abnormalities
N = 6,520†
N = 6,535†
Platelets Decreased
0.7
0.3
Adverse Effects
N = 6,492‡
N = 6,484‡
Other Toxicities
Mood
11.6
10.8
Infection/Sepsis
6.0
5.1
Constipation
4.4
3.2
Alopecia
5.2
4.4
Skin
5.6
4.7
Allergy
2.5
2.1
In the NSABP P-1 trial, 15.0% and 9.7% of participants receiving tamoxifen and placebo therapy, respectively withdrew from the trial for medical reasons. The following are the medical reasons for withdrawing from tamoxifen and placebo therapy, respectively: hot flashes (3.1% vs. 1.5%) and vaginal discharge (0.5% vs. 0.1%).
In the NSABP P-1 trial, 8.7% and 9.6% of participants receiving tamoxifen and placebo therapy, respectively withdrew for non-medical reasons.
On the NSABP P-1 trial, hot flashes of any severity occurred in 68% of women on placebo and in 80% of women on tamoxifen. Severe hot flashes occurred in 28% of women on placebo and 45% of women on tamoxifen. Vaginal discharge occurred in 35% and 55% of women on placebo and tamoxifen respectively; and was severe in 4.5% and 12.3% respectively. There was no difference in the incidence of vaginal bleeding between treatment arms.
Pediatric Patients
McCune-Albright Syndrome
Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted in adults treated with tamoxifen (see BOXED WARNING), continued monitoring of McCune-Albright patients treated with tamoxifen for long-term effects is recommended. The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond 1 year of treatment. The long-term effects of tamoxifen therapy in girls have not been established.
Postmarketing Experience
Less frequently reported adverse reactions are vaginal bleeding, vaginal discharge, menstrual irregularities, skin rash and headaches. Usually these have not been of sufficient severity to require dosage reduction or discontinuation of treatment. Very rare reports of erythema multiforme, Stevens-Johnson syndrome, bullous pemphigoid, interstitial pneumonitis, and rare reports of hypersensitivity reactions including angioedema have been reported with tamoxifen therapy. In some of these cases, the time to onset was more than one year. Rarely, elevation of serum triglyceride levels, in some cases with pancreatitis, may be associated with the use of tamoxifen (see PRECAUTIONS, Drug/Laboratory Testing Interactions).
-
OVERDOSAGE
Signs observed at the highest doses following studies to determine LD50 in animals were respiratory difficulties and convulsions.
Acute overdosage in humans has not been reported. In a study of advanced metastatic cancer patients which specifically determined the maximum tolerated dose of tamoxifen in evaluating the use of very high doses to reverse multidrug resistance, acute neurotoxicity manifested by tremor, hyperreflexia, unsteady gait and dizziness were noted. These symptoms occurred within 3 to 5 days of beginning tamoxifen and cleared within 2 to 5 days after stopping therapy. No permanent neurologic toxicity was noted. One patient experienced a seizure several days after tamoxifen was discontinued and neurotoxic symptoms had resolved. The causal relationship of the seizure to tamoxifen therapy is unknown. Doses given in these patients were all greater than 400 mg/m2 loading dose, followed by maintenance doses of 150 mg/m2 of tamoxifen given twice a day.
In the same study, prolongation of the QT interval on the electrocardiogram was noted when patients were given doses higher than 250 mg/m2 loading dose, followed by maintenance doses of 80 mg/m2 of tamoxifen given twice a day. For a woman with a body surface area of 1.5 m2 the minimal loading dose and maintenance doses given at which neurological symptoms and QT changes occurred were at least 6 fold higher in respect to the maximum recommended dose.
No specific treatment for overdosage is known; treatment must be symptomatic.
-
DOSAGE AND ADMINISTRATION
For patients with breast cancer, the recommended daily dose is 20 to 40 mg. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
In three single agent adjuvant studies in women, one 10 mg tamoxifen citrate tablet was administered two (ECOG and NATO) or three (Toronto) times a day for two years. In the NSABP B-14 adjuvant study in women with node-negative breast cancer, one 10 mg tamoxifen citrate tablet was given twice a day for at least 5 years. Results of the B-14 study suggest that continuation of therapy beyond five years does not provide additional benefit (see CLINICAL PHARMACOLOGY). In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy. There was no indication that doses greater than 20 mg per day were more effective. Current data from clinical trials support 5 years of adjuvant tamoxifen therapy for patients with breast cancer.
-
HOW SUPPLIED
Tamoxifen citrate tablets USP, 10 mg (base) are white, round, biconvex, film-coated, unscored tablets debossed "93" and "784" and are supplied in bottles of 60, 180, 500, and 1000. (NDC: 51862-447-60, NDC: 51862-447-18, NDC: 51862-447-05, NDC: 51862-447-10)
Tamoxifen citrate tablets USP, 20 mg (base) are white to off-white, round, biconvex, film-coated, unscored tablets debossed "93" and "782" and are supplied in bottles of 30, 100, 500, and 1000. (NDC: 51862-446-30, NDC: 51862-446-01, NDC: 51862-446-05, NDC: 51862-446-10)
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a well-closed, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Manufactured In Israel By:
TEVA PHARMACEUTICAL IND. LTD.
Jerusalem, 9777402, Israel
Distributed by:
Mayne Pharma
Greenville, NC 27834
Rev. M 7/2016
-
MEDICATION GUIDE
Tamoxifen (TA mox i fen) Citrate Tablets USP
Rx only
Written for women who use tamoxifen citrate tablets to lower their high chance of getting breast cancer or who have ductal carcinoma in situ (DCIS)
This Medication Guide discusses only the use of tamoxifen citrate tablets to lower the chance of getting breast cancer in high-risk women and in women treated for DCIS.
People taking tamoxifen citrate tablets to treat breast cancer have different benefits and different decisions to make than high-risk women or women with ductal carcinoma in situ (DCIS) taking tamoxifen citrate tablets to reduce the chance of getting breast cancer. If you already have breast cancer, talk with your doctor about how the benefits of treating breast cancer with tamoxifen citrate tablets compare to the risks that are described in this document.
Why should I read this Medication Guide?
This guide has information to help you decide whether to use tamoxifen citrate tablets to lower your chance of getting breast cancer.
You and your doctor should talk about whether the possible benefit of tamoxifen citrate tablets in lowering your high chance of getting breast cancer is greater than its possible risks. Your doctor has a special computer program or hand-held calculator to tell if you are in the high-risk group. If you have DCIS and have been treated with surgery and radiation therapy, your doctor may prescribe tamoxifen citrate tablets to decrease your chance of getting invasive (spreading) breast cancer.
Read this guide carefully before you start tamoxifen citrate tablets. It is important to read the information you get each time you get more medicine. There may be something new. This guide does not tell you everything about tamoxifen citrate tablets and does not take the place of talking with your doctor.
Only you and your doctor can determine if tamoxifen citrate tablets are right for you.
What is the most important information I should know about using tamoxifen citrate tablets to reduce the chance of getting breast cancer?
Tamoxifen citrate tablets are a prescription medicine that is like estrogen (female hormone) in some ways and different in other ways. In the breast, tamoxifen citrate tablets can block estrogen's effects. Because it does this, tamoxifen citrate tablets may block the growth of breast cancers that need estrogen to grow (cancers that are estrogen- or progesterone-receptor positive).
Tamoxifen citrate tablets can lower the chance of getting breast cancer in women with a higher than normal chance of getting breast cancer in the next five years (high-risk women) and women with DCIS. Because high-risk women don't have cancer yet, it is important to think carefully about whether the possible benefit of tamoxifen citrate tablets in lowering the chance of getting breast cancer is greater than its possible risks.
This Medication Guide reviews the risks and benefits of using tamoxifen citrate tablets to reduce the chance of getting breast cancer in high-risk women and women with DCIS. This guide does not discuss the special benefits and decisions for people who already have breast cancer.
Why do women and men use tamoxifen citrate tablets?
Tamoxifen citrate tablets have more than one use. Tamoxifen citrate tablets are used:
- 1. to lower the chance of getting breast cancer in women with a higher than normal chance of getting breast cancer in the next 5 years (high-risk women)
- 2. to lower the chance of getting invasive (spreading) breast cancer in women who had surgery and radiation for ductal carcinoma in situ (DCIS). DCIS means the cancer is only inside the milk ducts.
- 3. to treat breast cancer in women after they have finished early treatment. Early treatment can include surgery, radiation, and chemotherapy. Tamoxifen citrate tablets may keep the cancer from spreading to other parts of the body. It may also reduce the woman's chance of getting a new breast cancer.
- 4. in women and men, to treat breast cancer that has spread to other parts of the body (metastatic breast cancer).
This guide talks only about using tamoxifen citrate tablets to lower the chance of getting breast cancer (#1 and #2 above).
What are the benefits of tamoxifen citrate tablets to lower the chance of getting breast cancer in high-risk women and in women treated for DCIS?
A large U.S. study looked at high-risk women and compared the ones who took tamoxifen citrate tablets for 5 years with others who took a pill without tamoxifen citrate (placebo). High-risk women were defined as women who have a 1.7% or greater chance of getting breast cancer in the next 5 years, based on a special computer program. In this study:
- Out of every 1,000 high-risk women who took a placebo, each year about 7 got breast cancer.
- Out of every 1,000 high-risk women who took tamoxifen citrate tablets, each year about 4 got breast cancer.
The study showed that on average, high-risk women who took tamoxifen citrate tablets lowered their chances of getting breast cancer by 44%, from 7 in 1,000 to 4 in 1,000.
Another U.S. study looked at women with DCIS and compared those who took tamoxifen citrate tablets for 5 years with others who took a placebo. In this study:
- Out of every 1,000 women with DCIS who took placebo, each year about 17 got breast cancer.
- Out of every 1,000 women with DCIS who took tamoxifen citrate tablets, each year about 10 got breast cancer.
The study showed that on average, women with DCIS who took tamoxifen citrate tablets lowered their chances of getting invasive (spreading) breast cancer by 43%, from 17 in 1,000 to 10 in 1,000.
These studies do not mean that taking tamoxifen citrate tablets will lower your personal chance of getting breast cancer. We do not know what the benefits will be for any one woman who takes tamoxifen citrate tablets to reduce her chance of getting breast cancer.
What are the risks of tamoxifen citrate tablets?
In the studies described under "What are the benefits of tamoxifen citrate tablets?", the high-risk women who took tamoxifen citrate tablets got certain side effects at a higher rate than those who took a placebo. Some of these side effects can cause death.
In one study, in women who still had their uterus:
- Out of every 1,000 women who took a placebo, each year 1 got endometrial cancer (cancer of the lining of the uterus) and none got uterine sarcoma (cancer of the body of the uterus).
- Out of every 1,000 women who took tamoxifen citrate tablets, each year 2 got endometrial cancer and fewer than 1 got uterine sarcoma.
These results show that, on average, in high-risk women who still had their uterus, tamoxifen citrate tablets doubled the chance of getting endometrial cancer from 1 in 1,000 to 2 in 1,000, and it increased the chance of getting uterine sarcoma. This does not mean that taking tamoxifen citrate tablets will double your personal chance of getting endometrial cancer or increase your chance of getting uterine sarcoma. We do not know what this risk will be for any one woman. The risk is different for women who no longer have their uterus.
For all women in this study, taking tamoxifen citrate tablets increased the risk of having a blood clot in their lungs or veins, or of having a stroke. In some cases, women died from these effects.
Tamoxifen citrate tablets increased the risk of getting cataracts (clouding of the lens of the eye) or needing cataract surgery. (See "What are the possible side effects of tamoxifen citrate tablets?" for more details about side effects.)
What don't we know about taking tamoxifen citrate tablets to reduce the chance of getting breast cancer?
We don't know:
- if tamoxifen citrate tablets lower the chance of getting breast cancer in women who have abnormal breast cancer genes (BRCA1 and BRCA2)
- if taking tamoxifen citrate tablets for 5 years reduces the number of breast cancers a woman will get in her lifetime or if it only delays some breast cancers
- if tamoxifen citrate tablets help a woman live longer
- the effects of taking tamoxifen citrate tablets with hormone replacement therapy (HRT), birth control pills, or androgens (male hormones)
- the benefits of taking tamoxifen citrate tablets if you are less than 35 years old
Studies are being done to learn more about the long-term benefits and risks of using tamoxifen citrate tablets to reduce the chance of getting breast cancer.
What are the possible side effects of tamoxifen citrate tablets?
The most common side effect of tamoxifen citrate tablets is hot flashes. This is not a sign of a serious problem.
The next most common side effect is vaginal discharge. If the discharge is bloody, it could be a sign of a serious problem. [See "Changes in the lining (endometrium) or body of your uterus" below.]
Less common but serious side effects of tamoxifen citrate tablets are listed below. These can occur at any time. Call your doctor right away if you have any signs of side effects listed below:
-
Changes in the lining (endometrium) or body of your uterus. These changes may mean serious problems are starting, including cancer of the uterus. The signs of changes in the uterus are:
- Vaginal bleeding or bloody discharge that could be a rusty or brown color. You should call your doctor even if only a small amount of bleeding occurs.
- Change in your monthly bleeding, such as in the amount or timing of bleeding or increased clotting.
- Pain or pressure in your pelvis (below your belly button).
-
Blood clots in your veins or lungs. These can cause serious problems, including death. You may get clots up to 2 to 3 months after you stop taking tamoxifen citrate tablets. The signs of blood clots are:
- sudden chest pain, shortness of breath, coughing up blood
- pain, tenderness, or swelling in one or both of your legs
-
Stroke. Stroke can cause serious medical problems, including death. The signs of stroke are:
- sudden weakness, tingling, or numbness in your face, arm or leg, especially on one side of your body
- sudden confusion, trouble speaking or understanding
- sudden trouble seeing in one or both eyes
- sudden trouble walking, dizziness, loss of balance or coordination
- sudden severe headache with no known cause
- Cataracts or increased chance of needing cataract surgery. The sign of these problems is slow blurring of your vision.
- Liver problems, including jaundice. The signs of liver problems include lack of appetite and yellowing of your skin or whites of your eyes.
These are not all the possible side effects of tamoxifen citrate tablets. For a complete list, ask your doctor or pharmacist.
Who should not take tamoxifen citrate tablets?
Do not take tamoxifen citrate tablets for any reason if you
- Are pregnant or plan to become pregnant while taking tamoxifen citrate tablets or during the 2 months after you stop taking tamoxifen citrate tablets. Tamoxifen citrate tablets may harm your unborn baby. It takes about 2 months to clear tamoxifen from your body. To be sure you are not pregnant, you can start taking tamoxifen citrate tablets while you are having your menstrual period. Or, you can take a pregnancy test to be sure you are not pregnant before you begin.
- Are breast feeding. We do not know if tamoxifen can pass through your milk and harm your baby.
- Have had an allergic reaction to tamoxifen or to any of its inactive ingredients.
If you get pregnant while taking tamoxifen citrate tablets, stop taking them right away and contact your doctor. Tamoxifen citrate tablets may harm your unborn baby.
Do not take tamoxifen citrate tablets to lower your chance of getting breast cancer if:
- You ever had a blood clot that needed medical treatment.
- You are taking medicines to thin your blood, like warfarin, (also called Coumadin®1).
- Your ability to move around is limited for most of your waking hours.
- You are at risk for blood clots. Your doctor can tell you if you are at high risk for blood clots.
- You do not have a higher than normal chance of getting breast cancer. Your doctor can tell you if you are a high-risk woman.
How should I take tamoxifen citrate tablets?
- Swallow the tablet(s) whole, with water or another non-alcoholic liquid. You can take tamoxifen citrate tablets with or without food. Take your medicine every day. It may be easier to remember if you take it at the same time each day.
- If you forget a dose, take it when you remember, then take the next dose as usual. If it is almost time for your next dose or you remember at your next dose, do not take extra tablets to make up the missed dose.
- Take tamoxifen citrate tablets for 5 years, unless your doctor tells you otherwise.
What should I avoid while taking tamoxifen citrate tablets?
- Do not become pregnant while taking tamoxifen citrate tablets or for 2 months after you stop. Tamoxifen citrate tablets can stop hormonal birth control methods from working. Hormonal methods include birth control pills, patches, injections, rings and implants. Therefore, while taking tamoxifen citrate tablets, use birth control methods that don't use hormones, such as condoms, diaphragms with spermicide, or plain IUDs. If you get pregnant, stop taking tamoxifen citrate tablets right away and call your doctor.
- Do not breast feed. We do not know if tamoxifen can pass through your milk and if it can harm the baby.
What should I do while taking tamoxifen citrate tablets?
- Have regular gynecology check-ups ("female exams"), breast exams and mammograms. Your doctor will tell you how often. These will check for signs of breast cancer and cancer of the endometrium (lining of the uterus). Because tamoxifen citrate tablets do not prevent all breast cancers, and you may get other types of cancers, you need these exams to find any cancers as early as possible.
- Because tamoxifen citrate tablets can cause serious side effects, pay close attention to your body. Signs you should look for are listed in "What are the possible side effects of tamoxifen citrate tablets?"
- Tell all of the doctors that you see that you are taking tamoxifen citrate tablets.
- Tell your doctor right away if you have any new breast lumps.
General information about the safe and effective use of tamoxifen citrate tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Your doctor has prescribed tamoxifen citrate tablets only for you. Do not give them to other people, even if they have a similar condition, because it may harm them. Do not use them for a condition for which they were not prescribed.
This Medication Guide is a summary of information about tamoxifen citrate tablets for women who use tamoxifen citrate tablets to lower their high chance of getting breast cancer or who have DCIS. If you want more information about tamoxifen citrate tablets, ask your doctor or pharmacist. They can give you information about tamoxifen citrate tablets that is written for health professionals. For more information about tamoxifen citrate tablets or breast cancer, call 1-844-825-8500.
Ingredients: tamoxifen citrate, USP, croscarmellose sodium, hypromellose, lactose (monohydrate), magnesium stearate, polyethylene glycol 400, povidone, corn starch, and titanium dioxide.
1 Coumadin® is a registered trademark of Bristol-Myers Squibb Pharmaceuticals.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured In Israel By:
TEVA PHARMACEUTICAL IND. LTD.
Jerusalem, 9777402, Israel
Distributed by:
Mayne Pharma
Greenville, NC 27834
Rev. F 7/2016
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
NDC: 51862-447-05
Tamoxifen Citrate
Tablets, USP
10 mg (base)*PHARMACIST: Dispense the accompanying
Medication Guide to each patient.Rx only
500 TABLETS

-
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label
NDC: 51862-446-05
Tamoxifen Citrate
Tablets, USP
20 mg (base)*PHARMACIST: Dispense the accompanying
Medication Guide to each patient.Rx only
500 TABLETS

-
INGREDIENTS AND APPEARANCE
TAMOXIFEN CITRATE
tamoxifen citrate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-447 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAMOXIFEN CITRATE (UNII: 7FRV7310N6) (TAMOXIFEN - UNII:094ZI81Y45) TAMOXIFEN 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code 93;784 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-447-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 2 NDC: 51862-447-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 3 NDC: 51862-447-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 4 NDC: 51862-447-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075797 08/03/2016 TAMOXIFEN CITRATE
tamoxifen citrate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-446 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAMOXIFEN CITRATE (UNII: 7FRV7310N6) (TAMOXIFEN - UNII:094ZI81Y45) TAMOXIFEN 20 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 9mm Flavor Imprint Code 93;782 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-446-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 2 NDC: 51862-446-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 3 NDC: 51862-446-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 4 NDC: 51862-446-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/03/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075797 08/03/2016 Labeler - Mayne Pharma Inc. (867220261) Establishment Name Address ID/FEI Business Operations Teva Pharmaceutical Industries Ltd. 533065814 MANUFACTURE(51862-447, 51862-446) , ANALYSIS(51862-447, 51862-446) , PACK(51862-447, 51862-446) , LABEL(51862-447, 51862-446)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.