CEFENIL RTU- ceftiofur hydrochloride suspension
Cefenil RTU by
Drug Labeling and Warnings
Cefenil RTU by is a Animal medication manufactured, distributed, or labeled by Aspen Veterinary Resources, Norbrook Laboratories Limited, Norbrook Manufacturing Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
For intramuscular and subcutaneous use in cattle and intramuscular use in swine. This product may be used in lactating dairy cattle. Not for use in calves to be processed for veal.
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits extra-label use of this drug in cattle and swine for disease prevention purposes; at unapproved doses, frequencies, durations, or routes of administration; and in unapproved major food producing species/production classes.
-
DESCRIPTION
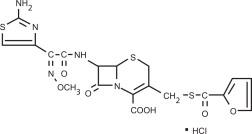
CEFENIL® RTU (ceftiofur hydrochloride sterile suspension) is a ready to use formulation that contains the hydrochloride salt of ceftiofur, which is a broad spectrum cephalosporin antibiotic. Each mL of this ready-to-use sterile suspension contains ceftiofur hydrochloride equivalent to 50 mg ceftiofur, 5.73 mg aluminum monostearate, 1.03 mg sorbitan monooleate and medium chain triglycerides.
Structure:
Figure 1
Chemical Name of Ceftiofur Hydrochloride: 5-Thia-1 -azabicyclo[4,2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(methoxyimino)-acetyl]amio]-3-[[(2-furanylcarbonyl) thio]methyl]-8-oxo-, hydrochloride salt [6R-[6α,7β(Z)]] –
-
INDICATIONS
Swine: CEFENIL RTU is indicated for treatment/control of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis and Streptococcus suis.
Cattle: CEFENIL RTU is indicated for treatment of the following bacterial diseases:
- - Bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni.
- - Acute bovine interdigital necrobacillosis (foot rot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
- - Acute metritis (0 to 14 days post-partum) associated with bacterial organisms susceptible to ceftiofur.
DOSAGE AND ADMINISTRATION
Shake for 90 seconds to ensure complete resuspension before using.
Swine: Administer intramuscularly at a dosage of 1.36 to 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW (1 mL of sterile suspension per 22 to 37 lb BW). Treatment should be repeated at 24 h intervals for a total of three consecutive days.
Cattle: - For bovine respiratory disease and acute bovine interdigitaI necrobaciIlosis: administer by intramuscular or subcutaneous administration at the dosage of 0.5 to 1.0 mg ceftiofur equivalents/lb (1.1 to 2.2 mg/kg) BW (1 to 2 mL sterile suspension per 100 lb BW). Administer daily at 24 h intervals for a total of three consecutive days. Additional treatments may be administered on Days 4 and 5 for animals which do not show a satisfactory response (not recovered) after the initial three treatments. In addition, for BRD only, administer intramuscularly or subcutaneously 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW every other day on Days 1 and 3 (48 h interval). Do not inject more than 15 mL per Injection site.
Selection of dosage level (0.5 to 1.0 mg/lb) and regimen/duration (daily or every other day for BRD only) should be based on an assessment of the severity of disease, pathogen susceptibility and clinical response.
- - For acute post-partum metritis: administer by intramuscular or subcutaneous administration at the dosage of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW (2 mL sterile suspension per 100 lb BW). Administer at 24 h intervals for five consecutive days. Do not inject more than 15 mL per injection site.
- CONTRAINDICATIONS
-
WARNINGS
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth, and clothing. Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention. The safety data sheet contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the safety datasheet (SDS), please call 1-866-591-5777.
-
RESIDUE WARNINGS:
Swine: When used according to label indications, dosage, and route of administration, treated swine must not be slaughtered for 4 days following the last treatment. Use of dosages in excess of those indicated or by unapproved routes of administration may result in illegal residues in edible tissues.
Cattle: When used according to label indications, dosage and route of administration, treated cattle must not be slaughtered for 3 days following the last treatment When used according to label indications, dosage and route of administration, a milk discard time is not required. Uses of dosages in excess of those indicated or by unapproved routes of administration, such as intramammary, may result in illegal residues in edible tissues and/or milk. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
-
PRECAUTIONS
The effects of ceftiofur on cattle and swine reproductive performance, pregnancy, and lactation have not been determined.
Swine: Areas of discoloration associated with the injection site at time periods of 11 days or less may result in trim-out of edible tissues at slaughter. The safety of ceftiofur has not been demonstrated for pregnant swine or swine intended for breeding.
Cattle: Following intramuscular or subcutaneous administration in the neck, areas of discoloration at the site may persist beyond 11 days resulting in trim loss of edible tissues at slaughter. Following intramuscular administration in the rear leg, areas of discoloration at the injection site may persist beyond 28 days resulting in trim loss of edible tissues at slaughter.
-
CLINICAL PHARMACOLOGY
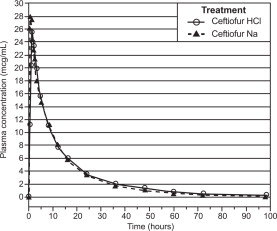
Swine: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to swine as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the labeled pathogens: Actinobacillus pleuropneumoniae, Pasteurella multocida, Streptococcus suis and Salmonella choleraesuis for the 24 hour (h) period between the dosing intervals. The MIC90 for Salmonella choleraesuis (1.0 μg/mL) is higher than the other three pathogens and plasma concentrations exceed this value for the entire dosing interval only after the 2.27 mg/lb (5.0 mg/kg) body weight (BW) dose.
Comparative Bioavailability Summary
Comparable plasma concentrations of ceftiofur administered as ceftiofur hydrochloride sterile suspension or ceftiofur sodium sterile solution were demonstrated after intramuscular administration of 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW. See Table 1 and Figure 2.
Table 1. Swine plasma concentrations and related parameters * of ceftiofur and desfuroyIceftiofur metabolites after ceftiofur hydrochloride sterile suspension, 50 mg/mL, or ceftiofur sodium sterile powder, 50 mg/mL, administered at 2.27 mg/lb ceftiofur equivalents /lb (5.0 mg/kg) BW IM.
Definitions:
Cmax - maximum plasma concentration in μg/mL.
tmax - the time after initial injection to when Cmax occurs, measured in hours.
AUC0-L0Q-the area under the plasma concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 μg/mL)
t1/2 - the plasma half life of the drug in hours.
C24 h - the concentration of drug at 24 h after administration.
C72 h - the concentration of drug at 72 h after administration.
t >0.2 h - the time (in hours) plasma concentrations remain above 0.2 μg/mL.
* Due to significant period effect and significant sequence effect in this study, data from period 1 only were used to evaluate these parameters.
Ceftiofur hydrochloride Ceftiofur sodium Cmax μg/mL: 26.1 ± 5.02 29.2 ± 5.01 tmax h: 0.66 - 2.0 (range) 0.33 - 2.0 (range) AUC0-L0Q μg h/mL: 321 ± 50.2 314 ± 55.1 t1/2 h: 16.2 ± 1.55 14.0 ± 1.23 C24 h μg/mL: 3.45 ± 0.431 3.53 ± 0.791 C72 h μg/mL: 0.518 ± 0.126 0.407 ± 0.0675 t >0.2 h: 93.8 ± 7.98 85.0 ± 7.71 Figure 2. Swine plasma concentrations of ceftiofur and desfuroylceftiofur metabolites after ceftiofur hydrochloride sterile suspension, 50 mg/mL, or ceftiofur sodium sterile powder, 50 mg/mL, were administered intramuscularly at 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW.
Concentrations of total ceftiofur in the lungs of pigs administered radiolabeled ceftiofur at 2.27 or 3.41 mg ceftiofur equivalents/lb (5.0 or 7.5 mg/kg) BW12 h after the last of three daily intramuscular injections at 24 h intervals averaged 3.66 and 5.63 μg/g.
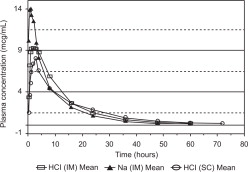
Cattle: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroyIceftiofur, the primary metabolite. Administration of ceftiofur to cattle as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroyIceftiofur metabolites in plasma above the MIC90 for the bovine respiratory disease (BRD) label pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni for at least 48 h. The relationship between plasma concentrations of ceftiofur and desfuroyIceftiofur metabolites above the MIC90 in plasma and efficacy has not been established for the treatment of bovine interdigital necrobacillosis (foot rot) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
Comparative Bioavailability Summary
The comparability of plasma concentrations of ceftiofur following administration of ceftiofur hydrochloride sterile suspension or ceftiofur sodium sterile solution was demonstrated after intramuscular or subcutaneous administration of ceftiofur hydrochloride and intramuscular administration of ceftiofur sodium at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW. See Table 2 and Figure 3.
Table 2. Cattle plasma concentrations and related parameters of ceftiofur and desfuroylceftiofur metabolites after ceftiofur hydrochloride sterile suspension, 50 mg/mL, administered intramuscularly or subcutaneously at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW and ceftiofur sodium sterile powder, 50 mg/mL, administered intramuscularly at 1.0 mg ceftiofur equivalents /lb (2.2 mg/kg) BW.
Definitions:
Cmax - maximum concentration of drug in plasma in μg/mL. tmax - the time after initial injection to when Cmax occurs, measured in hours. t>0.2 the time (in hours) plasma drug concentrations remain above 0.2 μg/mL. AUC0-L0Q -the area under the plasma drug concentrations vs. time curve from time of injection to the limit of quantitation of the assay (0.15 μg/mL). t1/2-the drug half life in plasma expressed in hours. C24 h - the plasma drug concentration 24 h after administration. C48 h - the plasma drug concentration 48 h after administration. *Values represent the separate means from each study.
Ceftiofur hydrochloride Ceftiofur sodium IM SC IM* Cmax μg/mL 11.0±1.69 8.56± 1.89 14.4-16.5 tmax h 1-4 (range) 1-5 (range) 0.33-3.0 t >0.2 h 60.5 ±6.27 51.0 ±6.53 50.7-50.9 AUC0-L0Q μg·h/mL 160 ±30.7 95.4 ±17.8 115-142 t1/2 h 12.0 ±2.63 11.5 ±2.57 9.50-11.1 C24 h μg/mL 1.47 ±0.380 0.926 ±0.257 0.86-1.16 C48 h μg/mL 0.340 ±0.110 0.271 ±0.086 0.250-0.268 Figure 3. Cattle plasma concentrations of ceftiofur and desfuroylceftiofur metabolites after ceftiofur hydrochloride sterile suspension, 50 mg/mL, was administered either intramuscularly or subcutaneously or ceftiofur sodium sterile powder, 50 mg/mL, was administered intramuscularly at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW.
Total residues of ceftiofur were measured in the lungs of cattle administered radiolabeled ceftiofur at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW at 24 h intervals for five consecutive days. Twelve h after the fifth injection of ceftiofur hydrochloride, total ceftiofur concentrations in the lung averaged 1.15 μg/g, while total ceftiofur concentrations in the lung 8 h after the fifth ceftiofur sodium injection averaged 1.18 μg/g.
-
CLINICAL MICROBIOLOGY
CEFENIL RTU is a ready to use formulation that contains the hydrochloride salt of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bacteriocidal, in vitro, resulting in inhibition of cell wall synthesis.
Swine: Studies with ceftiofur have demonstrated in vitro and in vivo activity against gram-negative pathogens, including Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis, and the gram-positive pathogen Streptococcus suis, all of which can be associated with swine bacterial respiratory disease - SRD (swine bacterial pneumonia). A summary of the minimum inhibitory concentration (MIC) values from SRD pathogens isolated from clinical field effectiveness studies is found in Table 3. Historic diagnostic laboratory MIC values for SRD pathogens from the US and Canada are found in Table 4.
Cattle: Studies with ceftiofur have demonstrated in vitro and in vivo activity against Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, the three major pathogenic bacteria associated with bovine respiratory disease (BRD, pneumonia, shipping fever), and against Fusobacterium necrophorum and Bacteroides melaninogenicus, two of the major pathogenic anaerobic bacteria associated with acute bovine interdigital necrobacillosis (foot rot, pododermatitis).
A summary of the MIC values for BRD and foot rot pathogens isolated from clinical field effectiveness studies is found in Table 3. Historic diagnostic MIC values for BRD and foot rot pathogens from the US and Canada are found in Table 4.
Antimicrobial Susceptibility
Summaries of MIC data are presented in Tables 3 and 4. Testing followed Clinical and Laboratory Standards Institute (CLSI) Guidelines.
Table 3. Ceftiofur MIC Values of Bacterial Isolates from Clinical Field Studies in the USA *Minimum inhibitory concentration (MIC) for 90% of the isolates
Animal Organism Number Tested Date Tested MIC90*( μg/mL) MIC Range (μg/mL) Bovine Mannheimia haemolytica 461 1988 1992 0.06 ≤0.03-0.13 Mannheimia haemolytica 42 1993 0.015 ≤0.003-0.03 Pasteurella multocida 318 1988-1992 0.06 ≤0.03-0.25 Pasteurella multocida 48 1993 ≤0.003 ≤0.003-0.015 Histophilus somni 109 1988-1992 0.06 ≤0.03-0.13 Histophilus somni 59 1993 ≤0.0019 no range Fusobacterium necrophorum 17 1994 ≤0.06 no range Swine Actinobacillus pleuropn. 83 1993 ≤0.03 ≤0.03-0.06 Pasteurella multocida 74 1993 ≤0.03 ≤0.03-0.06 Streptococcus suis 94 1993 0.25 ≤0.03-1.0 Salmonella choleraesuis 50 1993 1.0 1.0-2.0 beta-hemolytic Streptococcus spp. 24 1993 ≤0.03 ≤0.03-0.06 Actinobacillus suis 77 1998 0.0078 0.0019-0.0078 Haemophilus parasuis 76 1998 0.06 0.0039-0.25 Table 4. Ceftiofur MIC Values of Bacterial Isolates from Diagnostic Laboratories* in the USA and Canada Animal Organism Number Tested Date Tested MIC90**( μg/mL MIC Range (μg/mL) Bovine Mannheimia haemolytica 110 1997-1998 0.06 ≤0.03-0.25 Mannheimia haemolytica 139 1998-1999 ≤0.03 ≤0.03-0.5 Mannheimia haemolytica 209 1999-2000 ≤0.03 ≤0.03-0.12 Mannheimia haemolytica 189 2000-2001 ≤0.03 ≤0.03-0.12 Pasteurella multocida 107 1997-1998 <0.03 <0.03-0.25 Pasteurella multocida 181 1998-1999 ≤0.03 ≤0.03-0.5 Pasteurella multocida 208 1999-2000 ≤0.03 ≤0.03-0.12 Pasteurella multocida 259 2000-2001 <0.03 <0.03-0.12 Histophilus somni 48 1997-1998 ≤0.03 ≤0.03-0.25 Histophilus somni 87 1998-1999 ≤0.03 ≤0.03-0.125 Histophilus somni 77 1999-2000 ≤0.03 ≤0.03-0.06 Histophilus somni 129 2000-2001 ≤0.03 ≤0.03-0.12 Bacteroides fragilis group 29 1994 16.0 ≤0.06->16.0 Bacteroides spp., non-fragilis group 12 1994 16.0 0.13->16.0 Peptostreptococcus anaerobius 12 1994 2.0 0.13-2.0 Table 4. Continuation *The following in vitro data are available but their clinical significance is unknown.**Minimum inhibitory concentration(MIC) for 90%of the isolates
Animal Organism Number Tested Date Tested MIC90** (μg/mL MIC Range (μg/mL) Swine Actinobacillus pleuropn. 97 1997-1998 ≤0.03 no range Actinobacillus pleuropn. 111 1998-1999 ≤0.03 ≤0.03-0.25 Actinobacillus pleuropn. 126 1999-2000 ≤0.03 ≤0.03-0.06 Actinobacillus pleuropn. 89 2000-2001 ≤0.03 ≤0.03-0.06 Pasteurella multocida 114 1997-1998 ≤0.03 ≤0.03-1.0 Pasteurella multocida 147 1998-1999 ≤0.03 ≤0.03-0.5 Pasteurella multocida 173 1999-2000 ≤0.03 ≤0.03-0.06 Pasteurella multocida 186 2000-2001 ≤0.03 ≤0.03-0.12 Streptococcus suis 106 1997-1998 0.5 ≤0.03-4.0 Streptococcus suis 142 1998-1999 0.25 ≤0.03-1.0 Streptococcus suis 146 1999-2000 0.06 ≤0.03-4.0 Streptococcus suis 167 2000-2001 0.06 ≤0.03-4.0 Salmonella choleraesuis 96 1999-2000 1.0 0.03->4.0 Salmonella choleraesuis 101 2000-2001 1.0 0.5-2.0 Based on the pharmacokinetic studies of ceftiofur in swine and cattle after a single intramuscular injection of 1.36 to 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW (swine) or 0.5 to 1.0 mg ceftiofur equivalents/lb (1.1 to 2.2 mg/kg) BW (cattle) and the MIC and disk (30 μg) diffusion data, the following breakpoints are recommended by CLSI.
Zone Diameter (mm) MIC(μg/mL) Interpretation ≥21 ≤2.0 (S) Susceptible 18-20 4.0 (I) Intermediate ≤17 ≥8.0 (R) Resistant A report of "Susceptible" indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of "Intermediate" is a technical buffer zone and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. A report of "Resistant" indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected. Standardized procedures1 require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. The 30 μg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium standard reference powder (or disk) should provide the following MIC values for the reference strain. Ceftiofur sodium disks or powder reference standard is appropriate for both ceftiofur salts.
Table 5. Acceptable quality control ranges for ceftiofur against Clinical and Laboratory Standards Institute recommended American type Culture Collection (ATCC) reference strains *All testing performed using a 30 μg disk.
**Quality control ranges are applicable only to tests performed by disk diffusion test using a chocolate Mueller-Hinton agar, incubated in 5-7% CO2 for 20-24 hours.
***MIC quality control ranges are applicable only to tests performed by broth microdilution procedures using veterinary fastidious medium (VFM).
Organism name (ATCC No.) Zone diameter* (mm) MIC range (μg/mL) Escherichia coli (25922) 26-31 0.25-1.0 Staphylococcus aureus(29213) - 0.25-1.0 Staphylococcus aureus (25923) 27-31 - Pseudomonas aeruginosa (27853) 14-18 16.0-64.0 Actinobacillus pleuropneumoniae (27090) 34-42** 0.004-0.015*** Histophilus somni (700025) 36-46** 0.0005-0.004*** -
CLINICAL EFFICACY
Cattle: In addition to demonstrating comparable plasma concentrations, the following clinical efficacy data are provided.
A clinical study was conducted to evaluate the efficacy of ceftiofur hydrochloride administered subcutaneously for the treatment of the bacterial component of BRD under natural field conditions. When uniform clinical signs of BRD were present, 60 cattle (111 to 207 kg) were randomly assigned to one of the following treatment groups: negative control or ceftiofur hydrochloride at 0.5 or 1.0 ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW. Treatments were administered daily for three consecutive days.
Cattle were evaluated daily and animals that died or were euthanatized were necropsied and the lung lesions scored. On Day 15, all surviving animals were euthanatized and necropsied and the lung lesions scored. Mortality rates were 65%, 10% and 5% for negative controls, 0.5 mg ceftiofur equivalents/lb and 1.0 mg ceftiofur equivalents/lb, (1.1 or 2.2 mg/kg) BW, respectively. Mortality rates for both ceftiofur hydrochloride treatment groups were lower than for negative controls (P < 0.0001). Rectal temperatures 24 h after third treatment were 104.0°F, 103.1 °F and 102.8°F for negative controls, 0.5 mg/lb and 1.0 mg/lb (1.1 or 2.2 mg/kg) BW, respectively. The temperatures for both ceftiofur hydrochloride treatment groups were lower than the negative controls (P ≤ 0.05). Ceftiofur hydrochloride administered subcutaneously for three consecutive days at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW is an effective treatment for the bacterial component of BRD. A three-location clinical field study was conducted to evaluate the efficacy of ceftiofur hydrochloride administered intramuscularly daily for three days or every other day (Days 1 and 3) for the treatment of the bacterial component of naturally occurring BRD. When uniform signs of BRD were present, 360 beef crossbred cattle were randomly assigned to one of the following treatment groups: negative control, ceftiofur sodium at 0.5 mg ceftiofur equivalents/lb (1.1 mg/kg) BW daily for three days, ceftiofur hydrochloride at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW daily for three days, or ceftiofur hydrochloride at 1.0 mg ceftiofur equivalents/lb BW on Days 1 and 3 (every other day). All treatments were administered intramuscularly. All ceftiofur treatment groups (hydrochloride and sodium) and treatment regimens (every day and every other day) significantly (P<0.05) reduced Day 4 rectal temperature as compared to the negative control. Clinical success on Days 10 and 28 and mortality to Day 28 were not different for the ceftiofur groups (hydrochloride and sodium) and treatment regimens (every day and every other day). The results of this study demonstrate that daily and every other day (Days 1 and 3) intramuscular administration of ceftiofur hydrochloride are effective treatment regimens for the bacterial component of BRD. An eight location study was conducted under natural field conditions to evaluate the efficacy of ceftiofur hydrochloride for the treatment of acute post-partum metritis (0 to 14 days post-partum). When clinical signs of acute post-partum metritis (rectal temperature ≥103°F and fetid vaginal discharge) were observed, 361 lactating dairy cows were assigned randomly to treatment or negative control. Cattle were dosed either subcutaneously or intramuscularly, daily for five consecutive days.
On days 1,5 and 9 after the last day of dose administration, cows were evaluated for clinical signs of acute post-partum metritis. A cure was defined as rectal temperature <103°F and lack of fetid discharge. Cure rate for the 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW dose group was significantly improved relative to cure rate of the negative control on day 9. The results of this study demonstrate that ceftiofur hydrochloride administered daily for five consecutive days at a dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW is an effective treatment for acute post-partum metritis.
-
ANIMAL SAFETY
Swine: Results from a five-day tolerance study in normal feeder pigs indicated that ceftiofur sodium was well tolerated when administered at 57 mg ceftiofur equivalents/lb (125 mg/kg) (more than 25 times the highest recommended daily dosage of 2.27 mg/lb (5.0 mg/kg)) BW for five consecutive days. Ceftiofur administered intramuscularly to pigs produced no overt adverse signs of toxicity.
To determine the safety margin in swine, a safety/toxicity study was conducted. Five barrows and five gilts per group were administered ceftiofur sodium intramuscularly at 0, 2.27,6.81 and 11.36 mg ceftiofur equivalents/lb (0,5,15,25 mg/kg) BW for 15 days. This is 0,1,3 and 5 times the highest recommended dose of 2.27 mg/lb (5.0 mg/kg) BW/day and 5 times the recommended treatment length of 3 days. There were no adverse systemic effects observed, indicating that ceftiofur has a wide margin of safety when injected intramuscularly into feeder pigs at the highest recommended dose of 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW daily for 3 days or at levels up to 5 times the highest recommended dose for 5 times the recommended length of treatment
A separate study evaluated the injection site tissue tolerance of ceftiofur hydrochloride in swine when administered intramuscularly in the neck at 1.36 and 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW. Animals were necropsied at intervals to permit evaluations at 12 h, and 3,5,7,9,11,15,20, and 25 days after last injection. Injection sites were evaluated grossly at necropsy. No apparent changes (swelling or inflammation) were observed clinically after 12 h post-injection. Areas of discoloration associated with the injection site were observed at time periods less than 11 days after last injection.
Cattle: Results from a five-day tolerance study in feeder calves indicated that ceftiofur sodium was well tolerated at 25 times (25 mg ceftiofur equivalents/lb {55 mg/kg} BW) the highest recommended dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW for five consecutive days. Ceftiofur administered intramuscularly had no adverse systemic effects. In a 15-day safety/toxicity study, five steer and five heifer calves per group were administered ceftiofur sodium intramuscularly at 0 (vehicle control), 1,3, 5 and 10 times the highest recommended dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW to determine the safety factor.
There were no adverse systemic effects indicating that ceftiofur sodium has a wide margin of safety when injected intramuscularly into the feeder calves at 10 times (10 mg ceftiofur equivalents/lb {22 mg/kg} BW) the recommended dose for three times (15 days) the recommended length of treatment of three to five days. Local tissue tolerance to intramuscular injection of ceftiofur hydrochloride was evaluated in the following study. Results from a tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity in cattle when administered intramuscularly in the neck and rear leg at a dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW at each injection site. This represents a total dose per animal of 2.0 mg ceftiofur equivalents/lb (4.4 mg/kg) BW. Clinically noted changes (local swelling) at injection sites in the neck were very infrequent (2/48 sites) whereas noted changes in rear leg sites were more frequent (21/48 sites). These changes in the rear leg injection sites were generally evident on the day following injection and lasted from 1 to 11 days. At necropsy, injection sites were recognized by discoloration of the subcutaneous tissues and muscle that resolved in approximately 7 to 15 days in the neck and 19 to 28 days in the rear leg.
Results from another tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity to cattle when administered subcutaneously at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW at 24 h intervals for 5 days. Mild and usually transient, clinically visible or palpable reactions (local swelling) were localized at the injection site. At necropsy, injection sites were routinely recognized by edema, limited increase in thickness and color changes of the subcutaneous tissue and/or fascial surface of underlying muscle. The fascial surface of the muscle was visibly affected in most cases through 9.5 days after injection. Underlying muscle mass was not involved. There were no apparent differences in tissue response to administration of ceftiofur hydrochloride at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW.
-
TISSUE RESIDUE DEPLETION
Swine: A pivotal tissue residue decline study was conducted in swine. In this study, pigs received 2.27 mg of ceftiofur per lb body weight (5 mg of ceftiofur per kg body weight) per day for three consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 4 days after dosing. These data collectively support a 4-day pre-slaughter withdrawal period in swine when used according to label directions.
Cattle: Two pivotal tissue residue decline studies were conducted in cattle. In the first study, cattle received an intramuscular injection of 1.0 mg of ceftiofur per lb body weight (2.2 mg of ceftiofur per kg body weight) for five consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 3 days after dosing. In the second study, cattle received a subcutaneous injection of 1.0 mg of ceftiofur per lb body weight (2.2 mg of ceftiofur per kg body weight) for five consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 3 days after dosing. These data collectively support a 3-day pre-slaughter withdrawal period in cattle when used according to label directions. In addition, two blood-level bioequivalence studies were conducted in cattle (one using subcutaneous administration and one using intramuscular administration). Blood concentrations of ceftiofur (measured as ceftiofur free acid equivalents) were greater than the analytical method's limit of quantification through 12 hours after administration, and these data demonstrated bioequivalence between Cefenil® RTU and the referenced listed new animal drug. These data support a zero-day milk discard time in lactating dairy cows.
- STORAGE CONDITIONS
-
HOW SUPPLIED
CEFENIL RTU is available in 100 mL and 250 mL vials.
1 Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard - Second Edition. NCCLS document M31-A2. CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898,2002.
Approved by FDA under ANADA # 200-616
Made in the UK
Manufactured for: Aspen Veterinary Resources®, Ltd., Liberty, MO 64068, USA www.aspenveterinaryresources.com
®Cefenil is a registered trademark of Norbrook Laboratories Limited February 2020
-
Principal Display Panel – 250 mL Vial Label
NDC: 46066-938-04
aspen
VETERINARY RESOURCES,® LTD.CEFENIL® RTU
(ceftiofur hydrochloride
sterile suspension)Equivalent to 50 mg per mL
ceftiofur
For intramuscular and subcutaneous injection in cattle and intramuscular
injection in swine.This Product May Be Used In Lactating Dairy Cattle.
Not for use in calves to be processed for veal.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed
veterinarian. Federal law prohibits extra-label use of this drug in cattle and swine
for disease prevention purposes; at unapproved doses, frequencies,
durations, or routes of administration; and in unapproved major food
producing species/production classes.For Use In Animals Only.
Approved by FDA under ANADA # 200-616
NET CONTENTS:
250 mL -
INGREDIENTS AND APPEARANCE
CEFENIL RTU
ceftiofur hydrochloride suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-938 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ceftiofur hydrochloride (UNII: 6822A07436) (ceftiofur - UNII:83JL932I1C) ceftiofur hydrochloride 50 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-938-02 100 mL in 1 VIAL, GLASS 2 NDC: 46066-938-04 250 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200616 02/20/2020 Labeler - Aspen Veterinary Resources (627265361) Registrant - Norbrook Laboratories Limited (214580029) Establishment Name Address ID/FEI Business Operations Norbrook Manufacturing Ltd 986217040 MANUFACTURE, LABEL Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 211218325 LABEL Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 214580029 ANALYSIS
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.