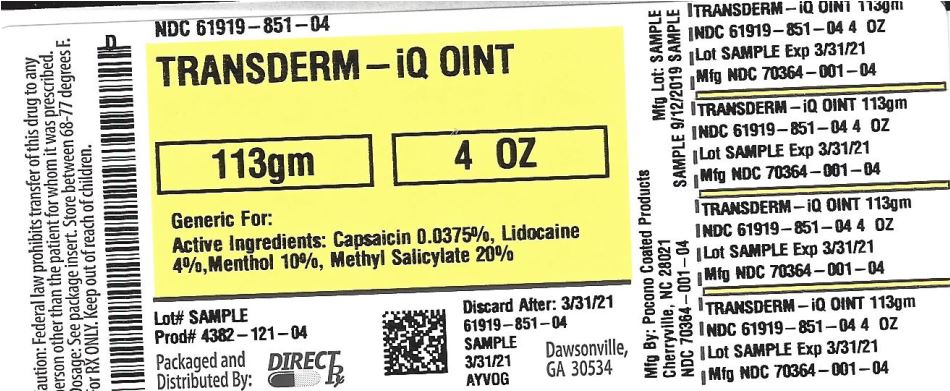
TRANSDERM-IQ by Direct_Rx TRANSDERM-IQ ointment
TRANSDERM-IQ by
Drug Labeling and Warnings
TRANSDERM-IQ by is a Otc medication manufactured, distributed, or labeled by Direct_Rx. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
WARNINGS
For external use only
use only as directed. Read and follow all directions and warnings on this carton.
avoid contact with eyes and mucous membranes
do not use at the same time as other topical analgesics
do not use on open wounds, cuts, damaged or infected skin
do not use with bandage or heating pad.Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days or clear up and occur again within a few daysIf pregnant or breast-feeding or if you have sensitive skin, ask a health professional before use
-
INACTIVE INGREDIENT
Purified Water, Mineral Oil, Isopropyl Myristate, Cetyl Alcohol, Glycerol Monostearate, Stearic Acid, Glycerin, Propylene Glycol, Cetereth-20, Carbomer, Aloe Vera, Disodium EDTA, Dimethicone, Petrolatum, Methylparaben, Triethanolamine, Diazolidinyl Urea, Iodopropynyl Butylcarbamate, Propylparaben, Beeswax, Sodium Carbomer, Phenonip, Triethanolamine. - SPL UNCLASSIFIED SECTION
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRANSDERM-IQ
transderm-iq ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61919-851(NDC:70364-001) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.0375 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 20 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CETYL ALCOHOL (UNII: 936JST6JCN) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIMETHICONE (UNII: 92RU3N3Y1O) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PETROLATUM (UNII: 4T6H12BN9U) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61919-851-04 113 g in 1 TUBE; Type 0: Not a Combination Product 07/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/25/2019 Labeler - Direct_Rx (079254320) Registrant - Direct_Rx (079254320) Establishment Name Address ID/FEI Business Operations Direct_Rx 079254320 repack(61919-851)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.