IBUPROFEN tablet, coated
IBUPROFEN by
Drug Labeling and Warnings
IBUPROFEN by is a Otc medication manufactured, distributed, or labeled by TARGET CORPORATION, TIME CAP LABORATORIES INC, MARKSANS PHARMA LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
DIRECTIONS
do not take more than directed
the smallest effective dose should be usedadults and children 12 years and older
take 1 tablet every 4 to 6 hours while symptoms persist
if pain or fever does not respond to 1 tablet, 2 tablets may be used
do not exceed 6 tablets in 24 hours, unless directed by a doctor.Children under 12 years
ask a doctor
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS
WARNINGS
Allergy alerts: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include: asthma (wheezing),blisters,facial swelling,hives,rash,shock,skin reddeningIf an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you: are age 60 or older; have had stomach ulcers or bleeding problems;take a blood-thinning (anticoagulant) or steroid drug; take other drug containing prescription NSAID (aspirin, ibuprofen, naproxen, or others)have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed.Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
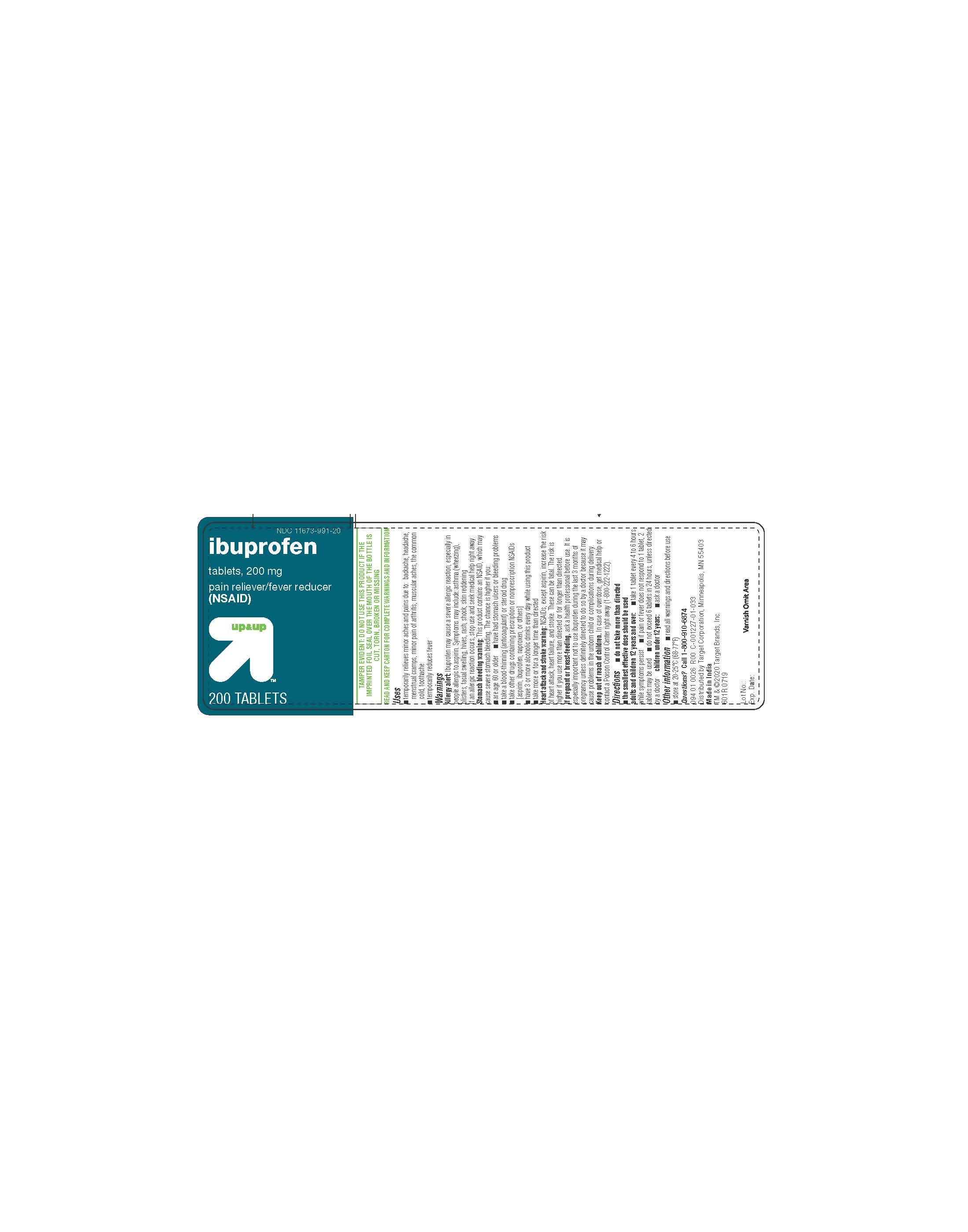
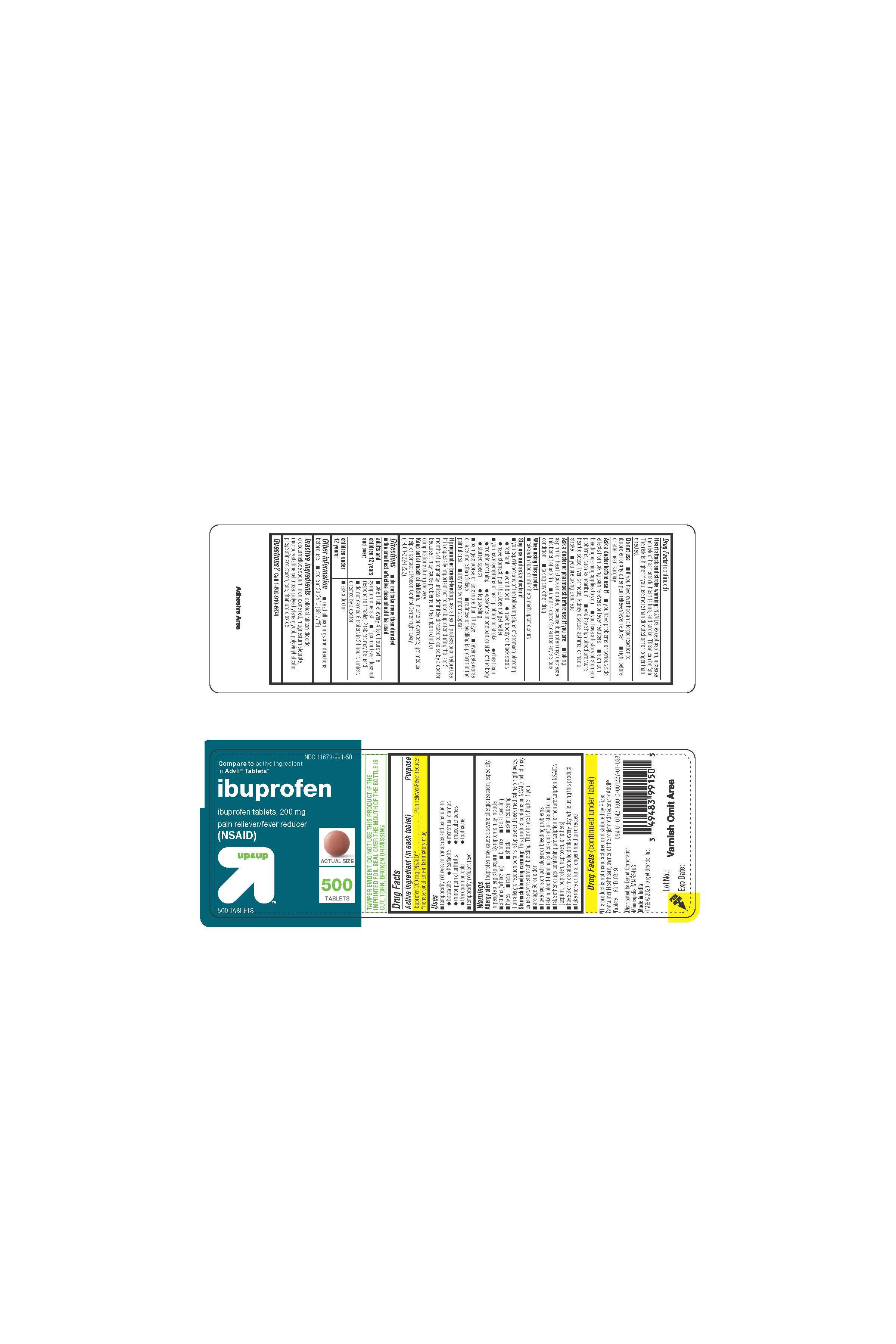
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11673-991 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code 114 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11673-991-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2020 2 NDC: 11673-991-05 50 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2020 3 NDC: 11673-991-20 200 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2020 4 NDC: 11673-991-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2020 5 NDC: 11673-991-88 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091239 02/04/2020 Labeler - TARGET CORPORATION (006961700) Registrant - TIME CAP LABORATORIES INC (037005209) Establishment Name Address ID/FEI Business Operations TIME CAP LABORATORIES, INC. 037052099 repack(11673-991) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LTD 925822975 manufacture(11673-991)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.