ESIKA- octinoxate and titanium dioxide powder
Esika by
Drug Labeling and Warnings
Esika by is a Otc medication manufactured, distributed, or labeled by Ventura International LTD, Bel Star S.A. (Colombia). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
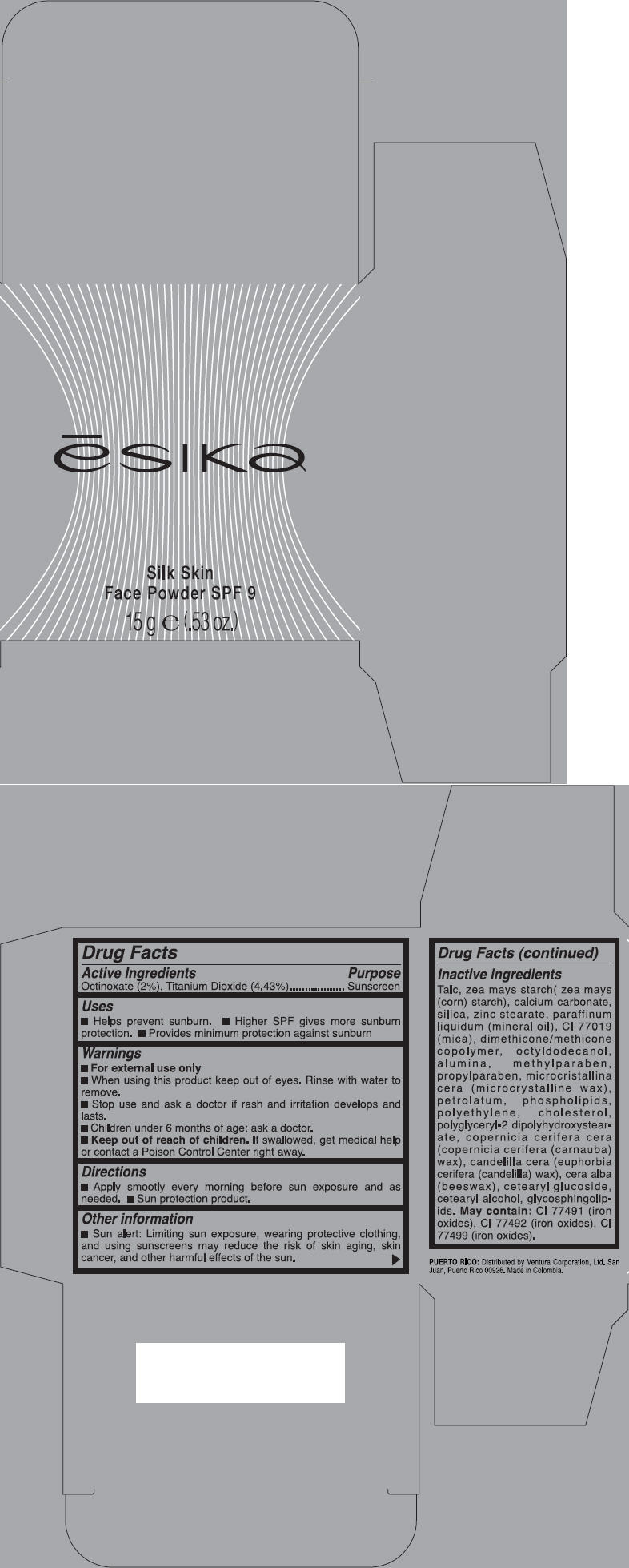
Talc, zea mays starch( zea mays (corn) starch), calcium carbonate, silica, zinc stearate, paraffinum liquidum (mineral oil), CI 77019 (mica), dimethicone/methicone copolymer, octyldodecanol, alumina, methylparaben, propylparaben, microcristallina cera (microcrystalline wax), petrolatum, phospholipids, polyethylene, cholesterol, polyglyceryl-2 dipolyhydroxystearate, copernicia cerifera cera (copernicia cerifera (carnauba) wax), candelilla cera (euphorbia cerifera (candelilla) wax), cera alba (beeswax), cetearyl glucoside, cetearyl alcohol, glycosphingolipids. May contain: CI 77491 (iron oxides), CI 77492 (iron oxides), CI 77499 (iron oxides).
- PRINCIPAL DISPLAY PANEL - 15 g Carton
-
INGREDIENTS AND APPEARANCE
ESIKA SILK SKIN
octinoxate and titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 14783-302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.02 g in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.044 g in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) CALCIUM CARBONATE (UNII: H0G9379FGK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC STEARATE (UNII: H92E6QA4FV) MINERAL OIL (UNII: T5L8T28FGP) MICA (UNII: V8A1AW0880) OCTYLDODECANOL (UNII: 461N1O614Y) ALUMINUM OXIDE (UNII: LMI26O6933) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) CHOLESTEROL (UNII: 97C5T2UQ7J) CARNAUBA WAX (UNII: R12CBM0EIZ) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14783-302-02 1 in 1 BOX 1 NDC: 14783-302-01 15 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/01/2011 Labeler - Ventura International LTD (603192787) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE
Trademark Results [Esika]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ESIKA 85527710 not registered Dead/Abandoned |
Ebel International Ltd. 2012-01-27 |
 ESIKA 78179029 not registered Dead/Abandoned |
Ebel International Ltd. 2002-10-28 |
 ESIKA 77225645 not registered Dead/Abandoned |
Ebel International Ltd. 2007-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.