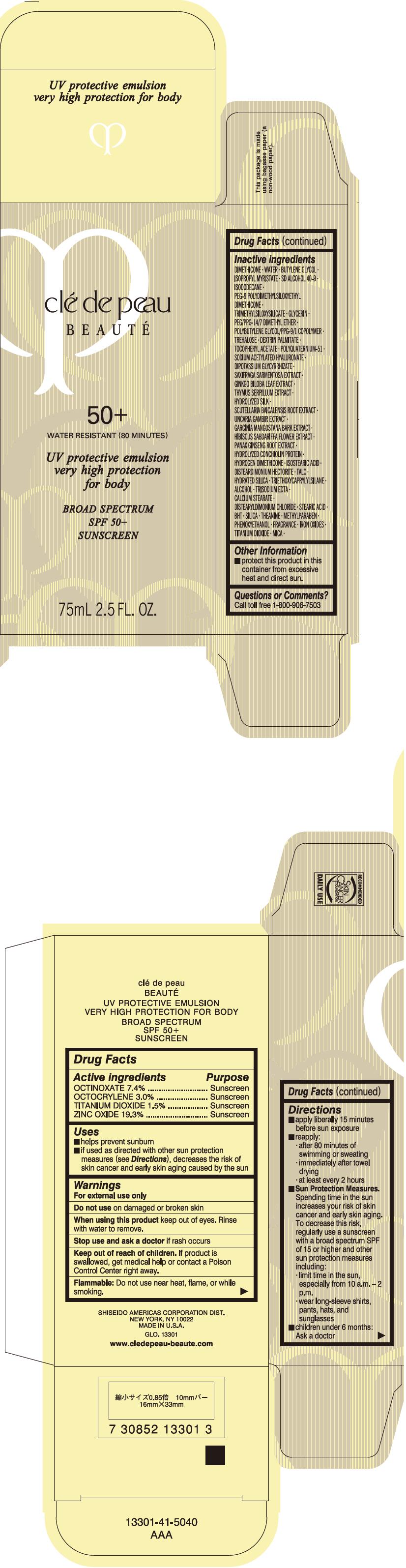
CLE DE PEAU BEAUTE UV PROTECTIVE VERY HIGH PROTECTION FOR BODY- octinoxate, octocrylene, titanium dioxide, and zinc oxide emulsion
CLE DE PEAU BEAUTE UV PROTECTIVE VERY HIGH PROTECTION FOR BODY by
Drug Labeling and Warnings
CLE DE PEAU BEAUTE UV PROTECTIVE VERY HIGH PROTECTION FOR BODY by is a Otc medication manufactured, distributed, or labeled by SHISEIDO AMERICAS CORPORATION, SHISEIDO AMERICA INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
DIMETHICONEWATERBUTYLENE GLYCOLISOPROPYL MYRISTATESD ALCOHOL 40-BISODODECANEPEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONETRIMETHYLSILOXYSILICATEGLYCERINPEG/PPG-14/7 DIMETHYL ETHERPOLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMERTREHALOSEDEXTRIN PALMITATETOCOPHERYL ACETATEPOLYQUATERNIUM-51SODIUM ACETYLATED HYALURONATEDIPOTASSIUM GLYCYRRHIZATESAXIFRAGA SARMENTOSA EXTRACTGINKGO BILOBA LEAF EXTRACTTHYMUS SERPILLUM EXTRACTHYDROLYZED SILKSCUTELLARIA BAICALENSIS ROOT EXTRACTUNCARIA GAMBIR EXTRACTGARCINIA MANGOSTANA BARK EXTRACTHIBISCUS SABDARIFFA FLOWER EXTRACTPANAX GINSENG ROOT EXTRACTHYDROLYZED CONCHIOLIN PROTEINHYDROGEN DIMETHICONEISOSTEARIC ACIDDISTEARDIMONIUM HECTORITETALCHYDRATED SILICATRIETHOXYCAPRYLYLSILANEALCOHOLTRISODIUM EDTACALCIUM STEARATEDISTEARYLDIMONIUM CHLORIDESTEARIC ACIDBHTSILICATHEANINEMETHYLPARABENPHENOXYETHANOLFRAGRANCEIRON OXIDESTITANIUM DIOXIDEMICA
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 75 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
CLE DE PEAU BEAUTE UV PROTECTIVE VERY HIGH PROTECTION FOR BODY
octinoxate, octocrylene, titanium dioxide, and zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58411-289 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5678 mg in 75 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2302 mg in 75 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1151 mg in 75 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14808 mg in 75 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISODODECANE (UNII: A8289P68Y2) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) TREHALOSE (UNII: B8WCK70T7I) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SAXIFRAGA STOLONIFERA LEAF (UNII: O3TMV4903H) GINKGO (UNII: 19FUJ2C58T) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) GARCINIA MANGOSTANA BARK (UNII: N6A4WY566I) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) ASIAN GINSENG (UNII: CUQ3A77YXI) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) ISOSTEARIC ACID (UNII: X33R8U0062) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TALC (UNII: 7SEV7J4R1U) HYDRATED SILICA (UNII: Y6O7T4G8P9) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALCOHOL (UNII: 3K9958V90M) EDETATE TRISODIUM (UNII: 420IP921MB) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) STEARIC ACID (UNII: 4ELV7Z65AP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58411-289-10 1 in 1 CARTON 02/01/2017 1 75 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2017 Labeler - SHISEIDO AMERICAS CORPORATION (193691821) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 manufacture(58411-289) , analysis(58411-289)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.