XIFAXAN- rifaximin tablet
XIFAXAN by
Drug Labeling and Warnings
XIFAXAN by is a Prescription medication manufactured, distributed, or labeled by Dispensing Solutions, Inc., PSS World Medical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XIFAXAN safely and effectively. See full prescribing information for XIFAXAN.
XIFAXAN® (rifaximin) Tablets
Initial U.S. Approval: 2004
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XIFAXAN and other antibacterial drugs, XIFAXAN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.INDICATIONS AND USAGE
XIFAXAN is a rifamycin antibacterial indicated for:
- The treatment of patients (≥ 12 years of age) with travelers’ diarrhea (TD) caused by noninvasive strains of Escherichia coli (1.1)
- Reduction in risk of overt hepatic encephalopathy (HE) recurrence in patients ≥ 18 years of age (1.2)
Limitations of Use
- TD: Do not use in patients with diarrhea complicated by fever or blood in the stool or diarrhea due to pathogens other than Escherichia coli (1.1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- 200 mg and 550 mg tablets (3)
CONTRAINDICATIONS
History of hypersensitivity to rifaximin, rifamycin antimicrobial agents, or any of the components of XIFAXAN (4.1)
WARNINGS AND PRECAUTIONS
- Travelers’ Diarrhea Not Caused by E. coli: XIFAXAN was not effective in diarrhea complicated by fever and/or blood in the stool or diarrhea due to pathogens other than E. coli. If diarrhea symptoms get worse or persist for more than 24-48 hours, discontinue XIFAXAN and consider alternative antibiotics (5.1)
- Clostridium difficile-Associated Diarrhea: Evaluate if diarrhea occurs after therapy or does not improve or worsens during therapy (5.2)
- Hepatic Impairment: Use with caution in patients with severe (Child-Pugh C) hepatic impairment (5.4, 8.7)
ADVERSE REACTIONS
- Most common adverse reactions in travelers’ diarrhea (≥ 5%): Flatulence, headache, abdominal pain, rectal tenesmus, defecation urgency and nausea (6.1)
- Most common adverse reactions in HE (≥ 10%): Peripheral edema, nausea, dizziness, fatigue, ascites, flatulence, and headache (6.1)
To report suspected adverse reactions, contact Salix Pharmaceuticals at 1-800-508-0024 and www.Salix.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2013
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Travelers’ Diarrhea
1.2 Hepatic Encephalopathy
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Travelers’ Diarrhea
2.2 Dosage for Hepatic Encephalopathy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Travelers’ Diarrhea Not Caused by Escherichia coli
5.2 Clostridium difficile-Associated Diarrhea
5.3 Development of Drug Resistant Bacteria
5.4 Severe (Child-Pugh C) Hepatic Impairment
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Travelers’ Diarrhea
14.2 Hepatic Encephalopathy
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Persistent Diarrhea
17.2 Clostridium difficile-Associated Diarrhea
17.3 Administration with Food
17.4 Antibacterial Resistance
17.5 Severe Hepatic Impairment
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XIFAXAN and other antibacterial drugs, XIFAXAN when used to treat infection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
1.1 Travelers’ Diarrhea
XIFAXAN 200 mg is indicated for the treatment of patients (≥ 12 years of age) with travelers’ diarrhea caused by noninvasive strains of Escherichia coli [see Warningsand Precautions (5), Clinical Pharmacology (12.4) and ClinicalStudies(14.1)].
Limitations of Use
XIFAXAN should not be used in patients with diarrhea complicated by fever or blood in the stool or diarrhea due to pathogens other than Escherichia coli.
1.2 Hepatic Encephalopathy
XIFAXAN 550 mg is indicated for reduction in risk of overt hepatic encephalopathy (HE) recurrence in patients ≥ 18 years of age.
In the trials of XIFAXAN for HE, 91% of the patients were using lactulose concomitantly. Differences in the treatment effect of those patients not using lactulose concomitantly could not be assessed.
XIFAXAN has not been studied in patients with MELD (Model for End-Stage Liver Disease) scores > 25, and only 8.6% of patients in the controlled trial had MELD scores over 19. There is increased systemic exposure in patients with more severe hepatic dysfunction [see Warnings and Precautions (5.4), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Travelers’ Diarrhea
The recommended dose of XIFAXAN is one 200 mg tablet taken orally three times a day for 3 days. XIFAXAN can be administered orally, with or without food [see Clinical Pharmacology (12.3)].
2.2 Dosage for Hepatic Encephalopathy
The recommended dose of XIFAXAN is one 550 mg tablet taken orally two times a day, with or without food[see Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
XIFAXAN is contraindicated in patients with a hypersensitivity to rifaximin, any of the rifamycin antimicrobial agents, or any of the components in XIFAXAN. Hypersensitivity reactions have included exfoliative dermatitis, angioneurotic edema, and anaphylaxis [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Travelers’ Diarrhea Not Caused by Escherichia coli
XIFAXAN was not found to be effective in patients with diarrhea complicated by fever and/or blood in the stool or diarrhea due to pathogens other than Escherichia coli.
Discontinue XIFAXAN if diarrhea symptoms get worse or persist more than 24-48 hours and alternative antibiotic therapy should be considered.
XIFAXAN is not effective in cases of travelers’ diarrhea due to Campylobacter jejuni. The effectiveness of XIFAXAN in travelers’ diarrhea caused by Shigella spp. and Salmonella spp. has not been proven. XIFAXAN should not be used in patients where Campylobacter jejuni, Shigella spp., or Salmonella spp. may be suspected as causative pathogens.
5.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon which may lead to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Development of Drug Resistant Bacteria
Prescribing XIFAXAN for travelers’ diarrhea in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.4 Severe (Child-Pugh C) Hepatic Impairment
There is increased systemic exposure in patients with severe hepatic impairment. Animal toxicity studies did not achieve systemic exposures that were seen in patients with severe hepatic impairment. The clinical trials were limited to patients with MELD scores <25. Therefore, caution should be exercised when administering XIFAXAN to patients with severe hepatic impairment (Child-Pugh C)
[see Use in Specific Populations (8.7), Nonclinical Toxicology (13.2) and Clinical Studies (14.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Travelers’ Diarrhea
The safety of XIFAXAN 200 mg taken three times a day was evaluated in patients with travelers’ diarrhea consisting of 320 patients in two placebo-controlled clinical trials with 95% of patients receiving three or four days of treatment with XIFAXAN. The population studied had a mean age of 31.3 (18-79) years of which approximately 3% were ≥ 65 years old, 53% were male and 84% were White, 11% were Hispanic.
Discontinuations due to adverse reactions occurred in 0.4% of patients. The adverse reactions leading to discontinuation were taste loss, dysentery, weight decrease, anorexia, nausea and nasal passage irritation.
All adverse reactions for XIFAXAN 200 mg three times daily that occurred at a frequency ≥ 2% in the two placebo-controlled trials combined are provided in Table 1. (These include adverse reactions that may be attributable to the underlying disease.)
Table 1. All Adverse Reactions With an Incidence ≥2% Among Patients Receiving XIFAXAN Tablets, 200 mg Three Times Daily, in Placebo-Controlled Studies MedDRA Preferred Term
Number (%) of Patients
XIFAXAN
Tablets, 600 mg/day
(N = 320)
Placebo
N = 228
*NOS: Not otherwise specified
Flatulence
36 (11%)
45 (20%)
Headache
31 (10%)
21 (9%)
Abdominal Pain NOS*
23 (7%)
23 (10%)
Rectal Tenesmus
23 (7%)
20 (9%)
Defecation Urgency
19 (6%)
21 (9%)
Nausea
17 (5%)
19 (8%)
Constipation
12 (4%)
8 (4%)
Pyrexia
10 (3%)
10 (4%)
Vomiting NOS
7 (2%)
4 (2%)
The following adverse reactions, presented by body system, have also been reported in <2% of patients taking XIFAXAN in the two placebo-controlled clinical trials where the 200 mg tablet was taken three times a day for travelers’ diarrhea. The following includes adverse reactions regardless of causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Lymphocytosis, monocytosis, neutropenia
Ear and Labyrinth Disorders: Ear pain, motion sickness, tinnitus
Gastrointestinal Disorders: Abdominal distension, diarrhea NOS, dry throat, fecal abnormality NOS, gingival disorder NOS, inguinal hernia NOS, dry lips, stomach discomfort
General Disorders and Administration Site Conditions: Chest pain, fatigue, malaise, pain NOS, weakness
Infections and Infestations: Dysentery NOS, respiratory tract infection NOS, upper respiratory tract infection NOS
Injury and Poisoning: Sunburn
Investigations: Aspartate aminotransferase increased, blood in stool, blood in urine, weight decreased
Metabolic and Nutritional Disorders: Anorexia, dehydration
Musculoskeletal, Connective Tissue, and Bone Disorders: Arthralgia, muscle spasms, myalgia, neck pain
Nervous System Disorders: Abnormal dreams, dizziness, migraine NOS, syncope, loss of taste
Psychiatric Disorders: Insomnia
Renal and Urinary Disorders: Choluria, dysuria, hematuria, polyuria, proteinuria, urinary frequency
Respiratory, Thoracic, and Mediastinal Disorders: Dyspnea NOS, nasal passage irritation, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, rhinitis NOS, rhinorrhea
Skin and Subcutaneous Tissue Disorders: Clamminess, rash NOS, sweating increased
Vascular Disorders: Hot flashes NOS
Hepatic Encephalopathy
The data described below reflect exposure to XIFAXAN 550 mg in 348 patients, including 265 exposed for 6 months and 202 exposed for more than a year (mean exposure was 364 days). The safety of XIFAXAN 550 mg taken two times a day for reducing the risk of overt hepatic encephalopathy recurrence in adult patients was evaluated in a 6-month placebo-controlled clinical trial (n = 140) and in a long term follow-up study (n = 280). The population studied had a mean age of 56.26 (range: 21-82) years; approximately 20% of the patients were ≥ 65 years old, 61% were male, 86% were White, and 4% were Black. Ninety-one percent of patients in the trial were taking lactulose concomitantly. All adverse reactions that occurred at an incidence ≥ 5% and at a higher incidence in XIFAXAN 550 mg-treated subjects than in the placebo group in the 6-month trial are provided in Table 2. (These include adverse events that may be attributable to the underlying disease).
Table 2: Adverse Reactions Occurring in ≥ 5% of Patients Receiving XIFAXAN and at a Higher Incidence Than Placebo
Number (%) of Patients
MedDRA Preferred TermXIFAXAN Tablets
550 mg TWICE
DAILY
N = 140
Placebo
N = 159Edema peripheral
21 (15%)
13 (8%)
Nausea
20 (14%)
21 (13%)
Dizziness
18 (13%)
13 (8%)
Fatigue
17 (12%)
18 (11%)
Ascites
16 (11%)
15 (9%)
Muscle spasms
13 (9%)
11 (7%)
Pruritus
13 (9%)
10 (6%)
Abdominal pain
12 (9%)
13 (8%)
Abdominal distension
11 (8%)
12 (8%)
Anemia
11 (8%)
6 (4%)
Cough
10 (7%)
11 (7%)
Depression
10 (7%)
8 (5%)
Insomnia
10 (7%)
11 (7%)
Nasopharyngitis
10 (7%)
10 (6%)
Abdominal pain upper
9 (6%)
8 (5%)
Arthralgia
9 (6%)
4 (3%)
Back pain
9 (6%)
10 (6%)
Constipation
9 (6%)
10 (6%)
Dyspnea
9 (6%)
7 (4%)
Pyrexia
9 (6%)
5 (3%)
Rash
7 (5%)
6 (4%)
The following adverse reactions, presented by body system, have also been reported in the placebo-controlled clinical trial in greater than 2% but less than 5% of patients taking XIFAXAN 550 mg taken orally two times a day for hepatic encephalopathy. The following includes adverse events occurring at a greater incidence than placebo, regardless of causal relationship to drug exposure.
Ear and Labyrinth Disorders: Vertigo
Gastrointestinal Disorders: Abdominal pain lower, abdominal tenderness, dry mouth, esophageal variceal bleed, stomach discomfort
General Disorders and Administration Site Conditions: Chest pain, generalized edema, influenza like illness, pain NOS
Infections and Infestations: Cellulitis, pneumonia, rhinitis, upper respiratory tract infection NOS
Injury, Poisoning and Procedural Complications: Contusion, fall, procedural pain
Investigations: Weight increased
Metabolic and Nutritional Disorders: Anorexia, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hyponatremia
Musculoskeletal, Connective Tissue, and Bone Disorders: Myalgia, pain in extremity
Nervous System Disorders: Amnesia, disturbance in attention, hypoesthesia, memory impairment, tremor
Psychiatric Disorders: Confusional state
Respiratory, Thoracic, and Mediastinal Disorders: Epistaxis
Vascular Disorders: Hypotension
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of XIFAXAN. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to XIFAXAN.
Infections and Infestations
Cases of C. difficile-associated colitis have been reported [see Warnings and Precautions (5.2)].
General
Hypersensitivity reactions, including exfoliative dermatitis, rash, angioneurotic edema (swelling of face and tongue and difficulty swallowing), urticaria, flushing, pruritus and anaphylaxis have been reported. These events occurred as early as within 15 minutes of drug administration.
-
7 DRUG INTERACTIONS
In vitro studies have shown that rifaximin did not inhibit cytochrome P450 isoenzymes 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and CYP3A4 at concentrations ranging from 2 to 200 ng/mL [see Clinical Pharmacology (12.3)]. Rifaximin is not expected to inhibit these enzymes in clinical use.
An in vitro study has suggested that rifaximin induces CYP3A4 [see Clinical Pharmacology (12.3)]. However, in patients with normal liver function, rifaximin at the recommended dosing regimen is not expected to induce CYP3A4. It is unknown whether rifaximin can have a significant effect on the pharmacokinetics of concomitant CYP3A4 substrates in patients with reduced liver function who have elevated rifaximin concentrations.
An in vitro study suggested that rifaximin is a substrate of P-glycoprotein. It is unknown whether concomitant drugs that inhibit P-glycoprotein can increase the systemic exposure of rifaximin [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well controlled studies in pregnant women. Rifaximin has been shown to be teratogenic in rats and rabbits at doses that caused maternal toxicity. XIFAXAN tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Administration of rifaximin to pregnant rats and rabbits at dose levels that caused reduced body weight gain resulted in eye malformations in both rat and rabbit fetuses. Additional malformations were observed in fetal rabbits that included cleft palate, lumbar scoliosis, brachygnathia, interventricular septal defect, and large atrium.
The fetal rat malformations were observed in a study of pregnant rats administered a high dose that resulted in 16 times the therapeutic dose to diarrheic patients or 1 times the therapeutic dose to patients with hepatic encephalopathy (based upon plasma AUC comparisons). Fetal rabbit malformations were observed from pregnant rabbits administered mid and high doses that resulted in 1 or 2 times the therapeutic dose to diarrheic patients or less than 0.1 times the dose in patients with hepatic encephalopathy, based upon plasma AUC comparisons.
Post-natal developmental effects were not observed in rat pups from pregnant/lactating female rats dosed during the period from gestation to Day 20 post-partum at the highest dose which resulted in approximately 16 times the human therapeutic dose for travelers’ diarrhea (based upon AUCs) or approximately 1 times the AUCs derived from therapeutic doses to patients with hepatic encephalopathy.
8.3 Nursing Mothers
It is not known whether rifaximin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from XIFAXAN, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of XIFAXAN 200 mg in pediatric patients with travelers’ diarrhea less than 12 years of age have not been established.
The safety and effectiveness of XIFAXAN 550 mg for HE have not been established in patients < 18 years of age.
8.5 Geriatric Use
Clinical studies with rifaximin 200 mg for travelers’ diarrhea did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger subjects.
In the controlled trial with XIFAXAN 550 mg for hepatic encephalopathy, 19.4% were 65 and over, while 2.3% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
8.7 Hepatic Impairment
Following administration of XIFAXAN 550 mg twice daily to patients with a history of hepatic encephalopathy, the systemic exposure (i.e., AUCτ) of rifaximin was about 10-, 13-, and 20-fold higher in those patients with mild (Child-Pugh A), moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment, respectively, compared to that in healthy volunteers. No dosage adjustment is recommended because rifaximin is presumably acting locally. Nonetheless, caution should be exercised when XIFAXAN is administered to patients with severe hepatic impairment
[see Warnings and Precautions (5.4), Clinical Pharmacology (12.3), Nonclinical Toxicology (13.2), and Clinical Studies (14.2)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage with XIFAXAN. In clinical studies at doses higher than the recommended dose (> 600 mg/day for travelers’ diarrhea or > 1100 mg/day for hepatic encephalopathy), adverse reactions were similar in subjects who received doses higher than the recommended dose and placebo. In the case of overdosage, discontinue XIFAXAN, treat symptomatically, and institute supportive measures as required.
-
11 DESCRIPTION
XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2S,16Z,18E,20S,21S,22R,23R,24R,25S,26S,27S,28E)-5,6,21,23,25-pentahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca-[1,11,13]trienimino)benzofuro[4,5-e]pyrido[1,2-á]-benzimidazole-1,15(2H)-dione,25-acetate. The empirical formula is C43H51N3O11 and its molecular weight is 785.9. The chemical structure is represented below:
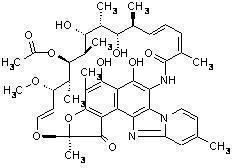
XIFAXAN Tablets for oral administration are film-coated and contain 200 mg or 550 mg of rifaximin.
Inactive ingredients:
Each 200 mg tablet contains colloidal silicon dioxide, disodium edetate, glycerol palmitostearate, hypromellose, microcrystalline cellulose, propylene glycol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
Each 550 mg tablet contains colloidal silicon dioxide, glycerol palmitostearate, microcrystalline cellulose, polyethylene glycol/macrogol, polyvinyl alcohol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
Travelers’ Diarrhea
Systemic absorption of rifaximin (200 mg three times daily) was evaluated in 13 subjects challenged with shigellosis on Days 1 and 3 of a three-day course of treatment. Rifaximin plasma concentrations and exposures were low and variable. There was no evidence of accumulation of rifaximin following repeated administration for 3 days (9 doses). Peak plasma rifaximin concentrations after 3 and 9 consecutive doses ranged from 0.81 to 3.4 ng/mL on Day 1 and 0.68 to 2.26 ng/mL on Day 3. Similarly, AUC0-last estimates were 6.95 ± 5.15 ngh/mL on Day 1 and 7.83 ± 4.94 ngh/mL on Day 3. XIFAXAN is not suitable for treating systemic bacterial infections because of limited systemic exposure after oral administration [see Warnings and Precautions (5.1)].
Hepatic Encephalopathy
After a single dose and multiple doses of rifaximin 550 mg in healthy subjects, the mean time to reach peak plasma concentrations was about an hour. The pharmacokinetic (PK) parameters were highly variable and the accumulation ratio based on AUC was 1.37.
The PK of rifaximin in patients with a history of HE was evaluated after administration of XIFAXAN, 550 mg two times a day. The PK parameters were associated with a high variability and mean rifaximin exposure (AUCτ) in patients with a history of HE (147 ngh/mL) was approximately 12-fold higher than that observed in healthy subjects following the same dosing regimen (12.3 ngh/mL). When PK parameters were analyzed based on Child-Pugh Class A, B, and C, the mean AUCτ was 10-, 13-, and 20-fold higher, respectively, compared to that in healthy subjects (Table 3).
Table 3. Mean (± SD) Pharmacokinetic Parameters of Rifaximin at Steady-State in Patients with a History of Hepatic Encephalopathy by Child-Pugh Class1
Healthy Subjects
(n = 14)
Child-Pugh Class
A (n = 18)
B (n = 7)
C (n = 4)
1Cross-study comparison with PK parameters in healthy subjects
2Median (range)AUCtau
(ngh/mL)
12.3 ± 4.8
118 ± 67.8
161 ± 101
246 ± 120
Cmax
(ng/mL)
3.4 ± 1.6
19.5 ± 11.4
25.1 ± 12.6
35.5 ± 12.5
Tmax2
(h)
0.8 (0.5, 4.0)
1 (0.9, 10)
1 (0.97, 1)
1 (0, 2)
Food Effect in Healthy Subjects
A high-fat meal consumed 30 minutes prior to XIFAXAN dosing in healthy subjects delayed the mean time to peak plasma concentration from 0.75 to 1.5 hours and increased the systemic exposure (AUC) of rifaximin by 2-fold (Table 4).
Table 4. Mean (± SD) Pharmacokinetic Parameters After Single-Dose Administration of XIFAXAN Tablets 550 mg in Healthy Subjects Under Fasting and Fed Conditions (N = 12) Parameter
Fasting
Fed
1 Median (range)
Cmax (ng/mL)
4.1 ± 1.5
4.8 ± 4.3
Tmax1 (h)
0.8 (0.5, 2.1)
1.5 (0.5, 4.1)
Half-Life (h)
1.8 ± 1.4
4.8 ± 1.3
AUC (ngh/mL)
11.1 ± 4.2
22.5 ± 12
XIFAXAN can be administered with or without food [see Dosage and Administration (2.1 and 2.2)].
Distribution
Rifaximin is moderately bound to human plasma proteins. In vivo, the mean protein binding ratio was 67.5% in healthy subjects and 62% in patients with hepatic impairment when XIFAXAN 550 mg was administered.
Metabolism and Excretion
In a mass balance study, after administration of 400 mg 14C-rifaximin orally to healthy volunteers, of the 96.94% total recovery, 96.62% of the administered radioactivity was recovered in feces almost exclusively as the unchanged drug and 0.32% was recovered in urine mostly as metabolites with 0.03% as the unchanged drug. Rifaximin accounted for 18% of radioactivity in plasma. This suggests that the absorbed rifaximin undergoes metabolism with minimal renal excretion of the unchanged drug. The enzymes responsible for metabolizing rifaximin are unknown.
In a separate study, rifaximin was detected in the bile after cholecystectomy in patients with intact gastrointestinal mucosa, suggesting biliary excretion of rifaximin.
Specific Populations
Hepatic Impairment
The systemic exposure of rifaximin was markedly elevated in patients with hepatic impairment compared to healthy subjects. The mean AUC in patients with Child-Pugh Class C hepatic impairment was 2-fold higher than in patients with Child-Pugh Class A hepatic impairment (see Table 3), [see Warnings and Precautions (5.4) and Use in Specific Populations (8.7)].
Renal Impairment
The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
Drug Interactions
In vitro drug interaction studies have shown that rifaximin, at concentrations ranging from 2 to 200 ng/mL, did not inhibit human hepatic cytochrome P450 isoenzymes 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and 3A4.
In an in vitro study, rifaximin was shown to induce CYP3A4 at the concentration of 0.2 µM.
An in vitro study suggests that rifaximin is a substrate of P-glycoprotein. In the presence of P-glycoprotein inhibitor verapamil, the efflux ratio of rifaximin was reduced greater than 50% in vitro. The effect of P-glycoprotein inhibition on rifaximin was not evaluated in vivo.
The inhibitory effect of rifaximin on P-gp transporter was observed in an in vitro study. The effect of rifaximin on P-gp transporter was not evaluated in vivo.
Midazolam
The effect of rifaximin 200 mg administered orally every 8 hours for 3 days and for 7 days on the pharmacokinetics of a single dose of either midazolam 2 mg intravenous or midazolam 6 mg orally was evaluated in healthy subjects. No significant difference was observed in the metrics of systemic exposure or elimination of intravenous or oral midazolam or its major metabolite, 1’-hydroxymidazolam, between midazolam alone or together with rifaximin. Therefore, rifaximin was not shown to significantly affect intestinal or hepatic CYP3A4 activity for the 200 mg three times a day dosing regimen.
After XIFAXAN 550 mg was administered three times a day for 7 days and 14 days to healthy subjects, the mean AUC of single midazolam 2 mg orally was 3.8% and 8.8% lower, respectively, than when midazolam was administered alone. The mean Cmax of midazolam was also decreased by 4-5% when XIFAXAN was administered for 7-14 days prior to midazolam administration. This degree of interaction is not considered clinically meaningful.
The effect of rifaximin on CYP3A4 in patients with impaired liver function who have elevated systemic exposure is not known.
Oral Contraceptives Containing 0.07 mg Ethinyl Estradiol and 0.5 mg Norgestimate
The oral contraceptive study utilized an open-label, crossover design in 28 healthy female subjects to determine if rifaximin 200 mg orally administered three times a day for 3 days (the dosing regimen for travelers’ diarrhea) altered the pharmacokinetics of a single dose of an oral contraceptive containing 0.07 mg ethinyl estradiol and 0.5 mg norgestimate. Results showed that the pharmacokinetics of single doses of ethinyl estradiol and norgestimate were not altered by rifaximin [seeDrug Interactions (7)].
Effect of rifaximin on oral contraceptives was not studied for XIFAXAN 550 mg twice a day, the dosing regimen for hepatic encephalopathy.
12.4 Microbiology
Mechanism of Action
Rifaximin is a non-aminoglycoside semi-synthetic antibacterial derived from rifamycin SV. Rifaximin acts by binding to the beta-subunit of bacterial DNA-dependent RNA polymerase resulting in inhibition of bacterial RNA synthesis.
Escherichia coli has been shown to develop resistance to rifaximin in vitro. However, the clinical significance of such an effect has not been studied.
Rifaximin is a structural analog of rifampin. Organisms with high rifaximin minimum inhibitory concentration (MIC) values also have elevated MIC values against rifampin. Cross-resistance between rifaximin and other classes of antimicrobials has not been studied.
Rifaximin has been shown to be active against the following pathogen in clinical studies of infectious diarrhea as described in the Indications and Usage (1) section: Escherichia coli (enterotoxigenic and enteroaggregative strains).
For HE, rifaximin is thought to have an effect on the gastrointestinal flora.
Susceptibility Tests
In vitro susceptibility testing was performed according to the National Committee for Clinical Laboratory Standards (NCCLS) agar dilution method M7-A6 [see References (15)]. However, the correlation between susceptibility testing and clinical outcome has not been determined.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Malignant schwannomas in the heart were significantly increased in male Crl:CD® (SD) rats that received rifaximin by oral gavage for two years at 150 to 250 mg/kg/day (doses equivalent to 2.4 to 4 times the recommended dose of 200 mg three times daily for travelers’ diarrhea, and equivalent to 1.3 to 2.2 times the recommended dose of 550 mg twice daily for hepatic encephalopathy, based on relative body surface area comparisons). There was no increase in tumors in Tg.rasH2 mice dosed orally with rifaximin for 26 weeks at 150 to 2000 mg/kg/day (doses equivalent to 1.2 to 16 times the recommended daily dose for travelers’ diarrhea and equivalent to 0.7 to 9 times the recommended daily dose for hepatic encephalopathy, based on relative body surface area comparisons).
Rifaximin was not genotoxic in the bacterial reverse mutation assay, chromosomal aberration assay, rat bone marrow micronucleus assay, rat hepatocyte unscheduled DNA synthesis assay, or the CHO/HGPRT mutation assay. There was no effect on fertility in male or female rats following the administration of rifaximin at doses up to 300 mg/kg (approximately 5 times the clinical dose of 600 mg/day, and approximately 2.6 times the clinical dose of 1100 mg/day, adjusted for body surface area).
13.2 Animal Toxicology and/or Pharmacology
Oral administration of rifaximin for 3-6 months produced hepatic proliferation of connective tissue in rats (50 mg/kg/day) and fatty degeneration of liver in dogs (100 mg/kg/day). However, plasma drug levels were not measured in these studies. Subsequently, rifaximin was studied at doses as high as 300 mg/kg/day in rats for 6 months and 1000 mg/kg/day in dogs for 9 months, and no signs of hepatotoxicity were observed. The maximum plasma AUC 0-8 hr values from the 6 month rat and 9 month dog toxicity studies (range: 42-127 ngh/mL) was lower than the maximum plasma AUC 0-8 hr values in cirrhotic patients (range: 19-306 ngh/mL).
-
14 CLINICAL STUDIES
14.1 Travelers’ Diarrhea
The efficacy of XIFAXAN given as 200 mg orally taken three times a day for 3 days was evaluated in 2 randomized, multi‑center, double-blind, placebo-controlled studies in adult subjects with travelers’ diarrhea. One study was conducted at clinical sites in Mexico, Guatemala, and Kenya (Study 1). The other study was conducted in Mexico, Guatemala, Peru, and India (Study 2). Stool specimens were collected before treatment and 1 to 3 days following the end of treatment to identify enteric pathogens. The predominant pathogen in both studies was Escherichia coli.
The clinical efficacy of XIFAXAN was assessed by the time to return to normal, formed stools and resolution of symptoms. The primary efficacy endpoint was time to last unformed stool (TLUS) which was defined as the time to the last unformed stool passed, after which clinical cure was declared. Table 5 displays the median TLUS and the number of patients who achieved clinical cure for the intent to treat (ITT) population of Study 1. The duration of diarrhea was significantly shorter in patients treated with XIFAXAN than in the placebo group. More patients treated with XIFAXAN were classified as clinical cures than were those in the placebo group.
Table 5. Clinical Response in Study 1 (ITT population) XIFAXAN
(n=125)Placebo
(n=129)Estimate
(97.5% CI)
P-Valuea Hazard Ratio
b Difference in ratesMedian TLUS
(hours)32.5 58.6 1.78a
(1.26, 2.50)0.0002 Clinical cure, n
(%)99 (79.2) 78 (60.5) 18.7b
(5.3, 32.1)0.001 Microbiological eradication (defined as the absence of a baseline pathogen in culture of stool after 72 hours of therapy) rates for Study 1 are presented in Table 6 for patients with any pathogen at baseline and for the subset of patients with Escherichia coli at baseline. Escherichia coli was the only pathogen with sufficient numbers to allow comparisons between treatment groups.
Even though XIFAXAN had microbiologic activity similar to placebo, it demonstrated a clinically significant reduction in duration of diarrhea and a higher clinical cure rate than placebo. Therefore, patients should be managed based on clinical response to therapy rather than microbiologic response.
Table 6. Microbiologic Eradication Rates in Study 1 Subjects with a Baseline Pathogen XIFAXAN Placebo Overall 48/70 (68.6) 41/61 (67.2) E. coli 38/53 (71.7) 40/54 (74.1)
The results of Study 2 supported the results presented for Study 1. In addition, this study provided evidence that subjects treated with XIFAXAN with fever and/or blood in the stool at baseline had prolonged TLUS. These subjects had lower clinical cure rates than those without fever or blood in the stool at baseline. Many of the patients with fever and/or blood in the stool (dysentery-like diarrheal syndromes) had invasive pathogens, primarily Campylobacter jejuni, isolated in the baseline stool.
Also in this study, the majority of the subjects treated with XIFAXAN who had Campylobacter jejuni isolated as a sole pathogen at baseline failed treatment and the resulting clinical cure rate for these patients was 23.5% (4/17). In addition to not being different from placebo, the microbiologic eradication rates for subjects with Campylobacter jejuni isolated at baseline were much lower than the eradication rates seen for Escherichia coli.
In an unrelated open-label, pharmacokinetic study of oral XIFAXAN 200 mg taken every 8 hours for 3 days, 15 adult subjects were challenged with Shigella flexneri 2a, of whom 13 developed diarrhea or dysentery and were treated with XIFAXAN. Although this open-label challenge trial was not adequate to assess the effectiveness of XIFAXAN in the treatment of shigellosis, the following observations were noted: eight subjects received rescue treatment with ciprofloxacin either because of lack of response to XIFAXAN treatment within 24 hours (2), or because they developed severe dysentery (5), or because of recurrence of Shigella flexneri in the stool (1); five of the 13 subjects received ciprofloxacin although they did not have evidence of severe disease or relapse.
14.2 Hepatic Encephalopathy
The efficacy of XIFAXAN 550 mg taken orally two times a day was evaluated in a randomized, placebo-controlled, double-blind, multi-center 6-month trial of adult subjects from the U.S., Canada and Russia who were defined as being in remission (Conn score of 0 or 1) from hepatic encephalopathy (HE). Eligible subjects had ≥ 2 episodes of HE associated with chronic liver disease in the previous 6 months.
A total of 299 subjects were randomized to receive either XIFAXAN (n=140) or placebo (n=159) in this study. Patients had a mean age of 56 years (range, 21-82 years), 81% < 65 years of age, 61% were male and 86% White. At baseline, 67% of patients had a Conn score of 0 and 68% had an asterixis grade of 0. Patients had MELD scores of either ≤ 10 (27%) or 11 to 18 (64%) at baseline. No patients were enrolled with a MELD score of > 25. Nine percent of the patients were Child-Pugh Class C. Lactulose was concomitantly used by 91% of the patients in each treatment arm of the study. Per the study protocol, patients were withdrawn from the study after experiencing a breakthrough HE episode. Other reasons for early study discontinuation included: adverse reactions (XIFAXAN 6%; placebo 4%), patient request to withdraw (XIFAXAN 4%; placebo 6%) and other (XIFAXAN 7%; placebo 5%).
The primary endpoint was the time to first breakthrough overt HE episode. A breakthrough overt HE episode was defined as a marked deterioration in neurological function and an increase of Conn score to Grade ≥ 2. In patients with a baseline Conn score of 0, a breakthrough overt HE episode was defined as an increase in Conn score of 1 and asterixis grade of 1.
Breakthrough overt HE episodes were experienced by 31 of 140 subjects (22%) in the XIFAXAN group and by 73 of 159 subjects (46%) in the placebo group during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE breakthrough by 58% during the 6-month treatment period. Presented below in Figure 1 is the Kaplan-Meier event-free curve for all subjects (n = 299) in the study.
Figure 1: Kaplan-Meier Event-Free Curves1 in HE Study (Time to First
Breakthrough-HE Episode up to 6 Months of Treatment, Day 170) (ITT
Population)
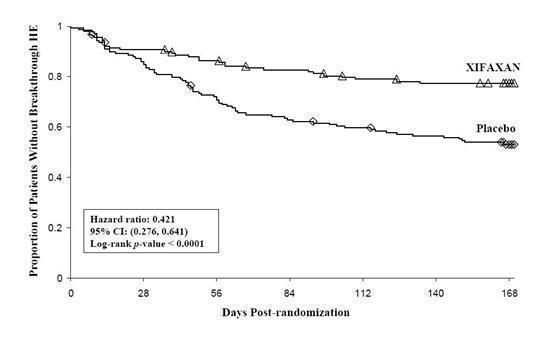
Note: Open diamonds and open triangles represent censored subjects.
1Event-free refers to non-occurrence of breakthrough HE.When the results were evaluated by the following demographic and baseline characteristics, the treatment effect of XIFAXAN 550 mg in reducing the risk of breakthrough overt HE recurrence was consistent for: sex, baseline Conn score, duration of current remission and diabetes. The differences in treatment effect could not be assessed in the following subpopulations due to small sample size: non-White (n=42), baseline MELD > 19 (n=26), Child-Pugh C (n=31), and those without concomitant lactulose use (n=26).
HE-related hospitalizations (hospitalizations directly resulting from HE, or hospitalizations complicated by HE) were reported for 19 of 140 subjects (14%) and 36 of 159 subjects (23%) in the XIFAXAN and placebo groups respectively. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE-related hospitalizations by 50% during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves is shown in Figure 2.
Figure 2: Kaplan-Meier Event-Free Curves1 in Pivotal HE Study (Time
to First HE-Related Hospitalization in HE Study up to 6 Months of
Treatment, Day 170) (ITT Population)
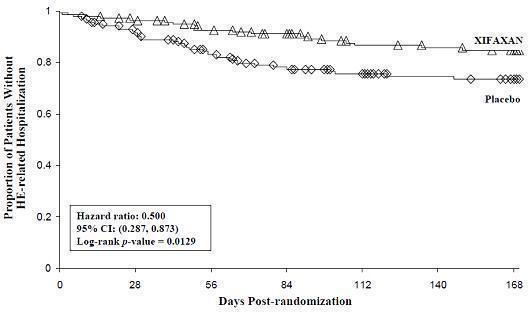
Note: Open diamonds and open triangles represent censored subjects.
1Event-free refers to non-occurrence of HE-related hospitalization. - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
200 mg tablet is a pink-colored, round, biconvex tablet with “Sx” debossed on one side. It is available in the following presentations:
- NDC: 65649-301-03, bottles of 30 tablets
- NDC: 65649-301-41, bottles of 100 tablets
- NDC: 65649-301-05, carton of 100 tablets, unit dose
The 550 mg tablet is a pink-colored, oval, biconvex tablet with “rfx” debossed on one side. It is available in the following presentations:
- NDC: 65649-303-02, bottles of 60 tablets
- NDC: 65649-303-03, carton of 60 tablets, unit dose
Storage
Store XIFAXAN Tablets at 20–25°C (68–77°F); excursions permitted to 15–30°C (59-86°F). See USP Controlled Room Temperature.
-
17 PATIENT COUNSELING INFORMATION
17.1 Persistent Diarrhea
For those patients being treated for travelers’ diarrhea, discontinue XIFAXAN if diarrhea persists more than 24-48 hours or worsens. Advise the patient to seek medical care for fever and/or blood in the stool [see Warnings and Precautions (5.1)].
17.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibiotics alters the normal flora of the colon which may lead to C. difficile. Patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If diarrhea occurs after therapy or does not improve or worsens during therapy, advise patients to contact a physician as soon as possible [see Warnings and Precautions (5.4)].
17.4 Antibacterial Resistance
Counsel patients that antibacterial drugs including XIFAXAN should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When XIFAXAN is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by XIFAXAN or other antibacterial drugs in the future.
17.5 Severe Hepatic Impairment
Patients should be informed that in patients with severe hepatic impairment (Child-Pugh C) there is an increase in systemic exposure to XIFAXAN [see Warnings and Precautions (5.4)].
Manufactured for:

Salix Pharmaceuticals, Inc.
Raleigh, NC 27615
XIFAXAN® is a trademark of Salix Pharmaceuticals, Inc., under license from Alfa Wassermann S.p.A.
Copyright © Salix Pharmaceuticals, Inc.Rifaximin for Travelers' Diarrhea and Hepatic encephalopathy is protected by US Patent Nos. 7,045,620; 7,612,199; 7,902,206 and 7,906,542. Rifaximin for Travelers' Diarrhea is also protected by US Patent No. 7,928,115.
Web site: www.Salix.com
All rights reserved.
VENART-156-2
OCT 2011
- PACKAGE LABEL - PRINCIPAL DISPLAY
-
INGREDIENTS AND APPEARANCE
XIFAXAN
rifaximin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68258-1107(NDC:65649-301) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIFAXIMIN (UNII: L36O5T016N) (RIFAXIMIN - UNII:L36O5T016N) RIFAXIMIN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL PALMITOSTEARATE (UNII: GSY51O183C) HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (PINK) Score no score Shape ROUND (BICONVEX) Size 10mm Flavor Imprint Code Sx Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68258-1107-1 10 in 1 BOTTLE 2 NDC: 68258-1107-2 28 in 1 BOTTLE 3 NDC: 68258-1107-4 14 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021361 07/25/2004 Labeler - Dispensing Solutions, Inc. (066070785) Registrant - PSS World Medical, Inc. (101822682) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel(68258-1107) , repack(68258-1107)
Trademark Results [XIFAXAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XIFAXAN 78428755 2965332 Live/Registered |
ALFASIGMA S.P.A. 2004-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
