CAFFEINE CITRATE injection, solution
Caffeine Citrate by
Drug Labeling and Warnings
Caffeine Citrate by is a Prescription medication manufactured, distributed, or labeled by AuroMedics Pharma LLC, Eugia Pharma Specialities Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Caffeine Citrate Injection USP
-
DESCRIPTION
Caffeine citrate injection USP for intravenous administration is clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution adjusted to pH 4.7. Each mL contains 20 mg caffeine citrate (equivalent to 10 mg of caffeine base) prepared in solution by the addition of 10 mg caffeine (anhydrous) USP to 5 mg citric acid monohydrate, 8.3 mg sodium citrate dihydrate and Water for Injection.
Caffeine (anhydrous) USP, a central nervous system stimulant, is an odorless white crystalline powder or granules, with a bitter taste. It is sparingly soluble in water and ethanol at room temperature. The chemical name of caffeine is 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione. In the presence of citric acid it forms caffeine citrate salt in solution. The structural formula and molecular weight of caffeine citrate follows.
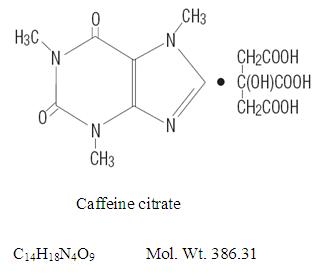
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Caffeine is structurally related to other methylxanthines, theophylline, and theobromine. It is a bronchial smooth muscle relaxant, a CNS stimulant, a cardiac muscle stimulant, and a diuretic.
Although the mechanism of action of caffeine in apnea of prematurity is not known, several mechanisms have been hypothesized. These include: (1) stimulation of the respiratory center, (2) increased minute ventilation, (3) decreased threshold to hypercapnia, (4) increased response to hypercapnia, (5) increased skeletal muscle tone, (6) decreased diaphragmatic fatigue, (7) increased metabolic rate, and (8) increased oxygen consumption.
Most of these effects have been attributed to antagonism of adenosine receptors, both A1 and A2 subtypes, by caffeine, which has been demonstrated in receptor binding assays and observed at concentrations approximating those achieved therapeutically.Pharmacokinetics
Absorption
After oral administration of 10 mg caffeine base/kg to preterm neonates, the peak plasma level (Cmax) for caffeine ranged from 6 to 10 mg/L and the mean time to reach peak concentration (Tmax) ranged from 30 minutes to 2 hours. The Tmax was not affected by formula feeding. The absolute bioavailability, however, was not fully examined in preterm neonates.
Distribution
Caffeine is rapidly distributed into the brain. Caffeine levels in the cerebrospinal fluid of preterm neonates approximate their plasma levels. The mean volume of distribution of caffeine in infants (0.8 to 0.9 L/kg) is slightly higher than that in adults (0.6 L/kg). Plasma protein binding data are not available for neonates or infants. In adults, the mean plasma protein binding in vitro is reported to be approximately 36%.
Metabolism
Hepatic cytochrome P4501A2 (CYP1A2) is involved in caffeine biotransformation. Caffeine metabolism in preterm neonates is limited due to their immature hepatic enzyme systems.
Interconversion between caffeine and theophylline has been reported in preterm neonates; caffeine levels are approximately 25% of theophylline levels after theophylline administration and approximately 3 to 8% of caffeine administered would be expected to convert to theophylline.
Elimination
In young infants, the elimination of caffeine is much slower than that in adults due to immature hepatic and/or renal function. Mean half-life (T1/2) and fraction excreted unchanged in urine (Ae) of caffeine in infants have been shown to be inversely related to gestational/postconceptual age. In neonates, the T1/2 is approximately 3 to 4 days and the Ae is approximately 86% (within 6 days). By 9 months of age, the metabolism of caffeine approximates that seen in adults (T1/2 = 5 hours and Ae = 1%).
Special Populations
Studies examining the pharmacokinetics of caffeine in neonates with hepatic or renal insufficiency have not been conducted. Caffeine citrate should be administered with caution in preterm neonates with impaired renal or hepatic function. Serum concentrations of caffeine should be monitored and dose administration of caffeine citrate should be adjusted to avoid toxicity in this population.Clinical Studies
One multicenter, randomized, double-blind trial compared caffeine citrate to placebo in eighty five (85) preterm infants (gestational age 28 to <33 weeks) with apnea of prematurity. Apnea of prematurity was defined as having at least 6 apnea episodes of greater than 20 seconds duration in a 24-hour period with no other identifiable cause of apnea. A 1 mL/kg (20 mg/kg caffeine citrate providing 10 mg/kg as caffeine base) loading dose of caffeine citrate was administered intravenously, followed by a 0.25 mL/kg (5 mg/kg caffeine citrate providing 2.5 mg/kg of caffeine base) daily maintenance dose administered either intravenously or orally (generally through a feeding tube). The duration of treatment in this study was limited to 10 to 12 days. The protocol allowed infants to be “rescued” with open-label caffeine citrate treatment if their apnea remained uncontrolled during the double-blind phase of the trial.
The percentage of patients without apnea on day 2 of treatment (24 to 48 hours after the loading dose) was significantly greater with caffeine citrate than placebo. The following table summarizes the clinically relevant endpoints evaluated in this study:
Caffeine Citrate
Placebo
p-value
Number of patients evaluated1
45
37
% of patients with zero apnea events on day 2
26.7
8.1
0.03
Apnea rate on day 2 (per 24 h)
4.9
7.2
0.134
% of patients with 50% reduction in apnea events from baseline on day 2
76
57
0.07
1 Of 85 patients who received drug, 3 were not included in the efficacy analysis because they had <6 apnea episodes/24 hours at baseline.
In this 10 to 12 day trial, the mean number of days with zero apnea events was 3 in the caffeine citrate group and 1.2 in the placebo group. The mean number of days with a 50% reduction from baseline in apnea events was 6.8 in the caffeine citrate group and 4.6 in the placebo group. - INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
During the double-blind, placebo-controlled clinical trial, 6 cases of necrotizing enterocolitis developed among the 85 infants studied (caffeine=46, placebo=39), with 3 cases resulting in death. Five of the six patients with necrotizing enterocolitis were randomized to or had been exposed to caffeine citrate.
Reports in the published literature have raised a question regarding the possible association between the use of methylxanthines and development of necrotizing enterocolitis, although a causal relationship between methylxanthine use and necrotizing enterocolitis has not been established. Therefore, as with all preterm infants, patients being treated with caffeine citrate should be carefully monitored for the development of necrotizing enterocolitis. -
PRECAUTIONS
General
Apnea of prematurity is a diagnosis of exclusion. Other causes of apnea (e.g., central nervous system disorders, primary lung disease, anemia, sepsis, metabolic disturbances, cardiovascular abnormalities, or obstructive apnea) should be ruled out or properly treated prior to initiation of caffeine citrate.
Caffeine is a central nervous system stimulant and in cases of caffeine overdose, seizures have been reported. Caffeine citrate should be used with caution in infants with seizure disorders.
The duration of treatment of apnea of prematurity in the placebo-controlled trial was limited to 10 to 12 days. The safety and efficacy of caffeine citrate for longer periods of treatment have not been established. Safety and efficacy of caffeine citrate for use in the prophylactic treatment of sudden infant death syndrome (SIDS) or prior to extubation in mechanically ventilated infants have also not been established.Cardiovascular
Although no cases of cardiac toxicity were reported in the placebo-controlled trial, caffeine has been shown to increase heart rate, left ventricular output, and stroke volume in published studies. Therefore, caffeine citrate should be used with caution in infants with cardiovascular disease.
Renal and Hepatic Systems
Caffeine citrate should be administered with caution in infants with impaired renal or hepatic function. Serum concentrations of caffeine should be monitored and dose administration of caffeine citrate should be adjusted to avoid toxicity in this population. (See CLINICAL PHARMACOLOGY, Elimination, Special Populations.)
Laboratory Tests
Prior to initiation of caffeine citrate, baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize theophylline to caffeine. Likewise, baseline serum levels of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery, since caffeine readily crosses the placenta.
In the placebo-controlled clinical trial, caffeine levels ranged from 8 to 40 mg/L. A therapeutic plasma concentration range of caffeine could not be determined from the placebo-controlled clinical trial. Serious toxicity has been reported in the literature when serum caffeine levels exceed 50 mg/L. Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity.
In clinical studies reported in the literature, cases of hypoglycemia and hyperglycemia have been observed. Therefore, serum glucose may need to be periodically monitored in infants receiving caffeine citrate.Interactions
Drug Interactions
Cytochrome P450 1A2 (CYP1A2) is known to be the major enzyme involved in the metabolism of caffeine. Therefore, caffeine has the potential to interact with drugs that are substrates for CYP1A2, inhibit CYP1A2, or induce CYP1A2.
Few data exist on drug interactions with caffeine in preterm neonates. Based on adult data, lower doses of caffeine may be needed following coadministration of drugs which are reported to decrease caffeine elimination (e.g., cimetidine and ketoconazole) and higher caffeine doses may be needed following coadministration of drugs that increase caffeine elimination (e.g., phenobarbital and phenytoin).
Caffeine administered concurrently with ketoprofen reduced the urine volume in four healthy volunteers. The clinical significance of this interaction in preterm neonates is not known.
Interconversion between caffeine and theophylline has been reported in preterm neonates. The concurrent use of these drugs is not recommended.Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year study in Sprague-Dawley rats, caffeine (as caffeine base) administered in drinking water was not carcinogenic in male rats at doses up to 102 mg/kg or in female rats at doses up to 170 mg/kg (approximately 2 and 4 times, respectively, the maximum recommended intravenous loading dose for infants on a mg/m2 basis). In an 18-month study in C57BL/6 mice, no evidence of tumorigenicity was seen at dietary doses up to 55 mg/kg (less than the maximum recommended intravenous loading dose for infants on a mg/m2 basis).
Caffeine (as caffeine base) increased the sister chromatid exchange (SCE) SCE/cell metaphase (exposure time dependent) in an in vivo mouse metaphase analysis. Caffeine also potentiated the genotoxicity of known mutagens and enhanced the micronuclei formation (5-fold) in folate-deficient mice. However, caffeine did not increase chromosomal aberrations in in vitro Chinese hamster ovary cell (CHO) and human lymphocyte assays and was not mutagenic in an in vitro CHO/hypoxanthine guanine phosphoribosyltransferase (HGPRT) gene mutation assay, except at cytotoxic concentrations. In addition, caffeine was not clastogenic in an in vivo mouse micronucleus assay.
Caffeine (as caffeine base) administered to male rats at 50 mg/kg/day subcutaneously (approximately equal to the maximum recommended intravenous loading dose for infants on a mg/m2 basis) for 4 days prior to mating with untreated females, caused decreased male reproductive performance in addition to causing embryotoxicity. In addition, long-term exposure to high oral doses of caffeine (3 g over 7 weeks) was toxic to rat testes as manifested by spermatogenic cell degeneration.Pregnancy
Pregnancy Category C
Concern for the teratogenicity of caffeine is not relevant when administered to infants. In studies performed in adult animals, caffeine (as caffeine base) administered to pregnant mice as sustained release pellets at 50 mg/kg (less than the maximum recommended intravenous loading dose for infants on a mg/m2 basis), during the period of organogenesis, caused a low incidence of cleft palate and exencephaly in the fetuses. There are no adequate and well-controlled studies in pregnant women. -
ADVERSE REACTIONS
Overall, the reported number of adverse events in the double-blind period of the controlled trial was similar for the caffeine citrate and placebo groups. The following table shows adverse events that occurred in the double-blind period of the controlled trial and that were more frequent in caffeine citrate-treated patients than placebo.
ADVERSE EVENTS THAT OCCURRED MORE FREQUENTLY IN CAFFEINE CITRATE-TREATED PATIENTS THAN PLACEBO DURING DOUBLE-BLIND THERAPY Adverse Event (AE)
Caffeine Citrate
N=46
n (%)
Placebo
N=39
n (%)
BODY AS A WHOLE
Accidental Injury
1 (2.2)
0 (0)
Feeding Intolerance
4 (8.7)
2 (5.1)
Sepsis
2 (4.3)
0 (0)
CARDIOVASCULAR SYSTEM
Hemorrhage
1 (2.2)
0 (0)
DIGESTIVE SYSTEM
Necrotizing Enterocolitis
2 (4.3)
1 (2.6)
Gastritis
1 (2.2)
0 (0)
Gastrointestinal Hemorrhage
1 (2.2)
0 (0)
HEMIC AND LYMPHATIC SYSTEM
Disseminated Intravascular Coagulation
1 (2.2)
0 (0)
METABOLIC AND NUTRITIVE DISORDERS
Acidosis
1 (2.2)
0 (0)
Healing Abnormal
1 (2.2)
0 (0)
NERVOUS SYSTEM
Cerebral Hemorrhage
1 (2.2)
0 (0)
RESPIRATORY SYSTEM
Dyspnea
1 (2.2)
0 (0)
Lung Edema
1 (2.2)
0 (0)
SKIN AND APPENDAGES
Dry Skin
1 (2.2)
0 (0)
Rash
4 (8.7)
3 (7.7)
Skin Breakdown
1 (2.2)
0 (0)
SPECIAL SENSES
Retinopathy of Prematurity
1 (2.2)
0 (0)
UROGENITAL SYSTEM
Kidney Failure
1 (2.2)
0 (0)
In addition to the cases above, three cases of necrotizing enterocolitis were diagnosed in patients receiving caffeine citrate during the open-label phase of the study.
Three of the infants who developed necrotizing enterocolitis during the trial died. All had been exposed to caffeine. Two were randomized to caffeine, and one placebo patient was “rescued” with open-label caffeine for uncontrolled apnea.
Adverse events described in the published literature include: central nervous system stimulation (i.e., irritability, restlessness, jitteriness), cardiovascular effects (i.e., tachycardia, increased left ventricular output, and increased stroke volume), gastrointestinal effects (i.e., increased gastric aspirate, gastrointestinal intolerance), alterations in serum glucose (i.e., hypoglycemia and hyperglycemia), and renal effects (i.e., increased urine flow rate, increased creatinine clearance, and increased sodium and calcium excretion). Published long-term follow-up studies have not shown caffeine to adversely affect neurological development or growth parameters.
-
OVERDOSAGE
Following overdose, serum caffeine levels have ranged from approximately 24 mg/L (a postmarketing spontaneous case report in which an infant exhibited irritability, poor feeding, and insomnia) to 350 mg/L. Serious toxicity has been associated with serum levels greater than 50 mg/L (see PRECAUTIONS – Laboratory Tests and DOSAGE AND ADMINISTRATION). Signs and symptoms reported in the literature after caffeine overdose in preterm infants include fever, tachypnea, jitteriness, insomnia, fine tremor of the extremities, hypertonia, opisthotonos, tonic-clonic movements, nonpurposeful jaw and lip movements, vomiting, hyperglycemia, elevated blood urea nitrogen, and elevated total leukocyte concentration. Seizures have also been reported in cases of overdose. One case of caffeine overdose complicated by development of intraventricular hemorrhage and long-term neurological sequelae has been reported. Another case of caffeine citrate overdose (from New Zealand) of an estimated 600 mg caffeine citrate (approximately 322 mg/kg) administered over 40 minutes was complicated by tachycardia, ST depression, respiratory distress, heart failure, gastric distention, acidosis, and a severe extravasation burn with tissue necrosis at the peripheral intravenous injection site. No deaths associated with caffeine overdose have been reported in preterm infants.
Treatment of caffeine overdose is primarily symptomatic and supportive. Caffeine levels have been shown to decrease after exchange transfusions. Convulsions may be treated with intravenous administration of diazepam or a barbiturate such as pentobarbital sodium. -
DOSAGE AND ADMINISTRATION
Prior to initiation of caffeine citrate injection USP, baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize theophylline to caffeine. Likewise, baseline serum levels of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery, since caffeine readily crosses the placenta.
The recommended loading dose and maintenance doses of caffeine citrate injection USP follow.
Dose of Caffeine Citrate Injection USP
Volume
Dose of Caffeine Citrate Injection USP
mg/kg
Route
Frequency
Loading Dose
1 mL/kg
20 mg/kg
Intravenous* (over 30 minutes)
One Time
Maintenance Dose
0.25 mL/kg
5 mg/kg
Intravenous*
(over 10 minutes) or Orally
Every 24 hours**
* using a syringe infusion pump
** beginning 24 hours after the loading dose
NOTE THAT THE DOSE OF CAFFEINE BASE IS ONE-HALF THE DOSE WHEN EXPRESSED AS CAFFEINE CITRATE (e.g., 20 mg of caffeine citrate is equivalent to 10 mg of caffeine base).
Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L.
Caffeine citrate injection USP should be inspected visually for particulate matter and discoloration prior to administration. Vials containing discolored solution or visible particulate matter should be discarded.Drug Compatibility
To test for drug compatibility with common intravenous solutions or medications, 20 mL of caffeine citrate injection USP were combined with 20 mL of a solution or medication, with the exception of an Intralipid® admixture, which was combined as 80 mL/80 mL. The physical appearance of the combined solutions was evaluated for precipitation. The admixtures were mixed for 10 minutes and then assayed for caffeine. The admixtures were then continually mixed for 24 hours, with further sampling for caffeine assays at 2, 4, 8, and 24 hours.
Based on this testing, caffeine citrate injection USP, 60 mg/3 mL is chemically stable for 24 hours at room temperature when combined with the following test products.
Dextrose Injection, USP 5%
50% Dextrose Injection USP
Intralipid® 20% IV Fat Emulsion
Aminosyn® 8.5% Crystalline Amino Acid Solution
Dopamine HCI Injection, USP 40 mg/mL diluted to 0.6 mg/mL with Dextrose Injection, USP 5%
Calcium Gluconate Injection, USP 10% (0.465 mEq/Ca+2/mL)
Heparin Sodium Injection, USP 1000 units/mL diluted to 1 unit/mL with Dextrose Injection, USP 5%
Fentanyl Citrate Injection, USP 50 mcg/mL diluted to 10 mcg/mL with Dextrose Injection, USP 5% -
HOW SUPPLIED
Caffeine citrate injection USP is available as clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution in 5 mL colorless glass vial. The vials of Caffeine citrate injection USP are sealed with a gray rubber stopper and an aluminum overseal with a white polypropylene disk inset.
The injection vial contains 3 mL solution at a concentration of 20 mg/mL caffeine citrate (60 mg/vial) equivalent to 10 mg/mL caffeine base (30 mg/vial).
3 mL Single Dose Vial,
individually packaged in a carton. NDC: 55150-187-03
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Preservative free. For single use only. Discard unused portion.
Intralipid® is a registered trademark of Fresenius Kabi AB.
Aminosyn® is a registered trademark of Hospira, Inc.
Distributed by:
AuroMedics Pharma LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad - 500038
India
Revised: March 2017 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 60 mg per 3 mL - Container Label
Rx only NDC: 55150-187-03
Caffeine
Citrate
Injection USP
60 mg per 3 mL
(20 mg / mL)
For Intravenous Use Only

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 60 mg per 3 mL - Container-Carton (1 Vial)
Rx only NDC: 55150-187-03
Caffeine
Citrate
Injection USP
60 mg per 3 mL
(20 mg / mL)
For Intravenous Use Only
For single use only;
discard unused portion.
3 mL Single Dose Vial
AUROMEDICS
-
INGREDIENTS AND APPEARANCE
CAFFEINE CITRATE
caffeine citrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55150-187 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE CITRATE (UNII: U26EO4675Q) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE CITRATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 5 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 8.3 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55150-187-03 1 in 1 CARTON 09/22/2015 1 3 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205013 09/22/2015 Labeler - AuroMedics Pharma LLC (968961354) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650498244 ANALYSIS(55150-187) , MANUFACTURE(55150-187)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.