COBENFY- xanomeline and trospium chloride capsule, coated pellets COBENFY- xanomeline and trospium chloride kit
Cobenfy by
Drug Labeling and Warnings
Cobenfy by is a Prescription medication manufactured, distributed, or labeled by E.R. Squibb & Sons, L.L.C.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COBENFY safely and effectively. See full prescribing information for COBENFY.
COBENFY™ (xanomeline and trospium chloride) capsules, for oral use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
COBENFY is a combination of xanomeline, a muscarinic agonist, and trospium chloride, a muscarinic antagonist, indicated for the treatment of schizophrenia in adults. (1)
DOSAGE AND ADMINISTRATION
- Assess liver enzymes and bilirubin prior to initiating treatment with COBENFY and as clinically indicated during treatment. (2.1)
- Assess heart rate at baseline and as clinically indicated during treatment with COBENFY. (2.1)
- Recommended starting dosage of COBENFY is 50 mg/20 mg orally twice daily for at least two days, then increase the dosage to 100 mg/20 mg twice daily for at least five days. (2.2)
- Dosage may be increased to 125 mg/30 mg orally twice daily based on patient tolerability and response. (2.2)
- See the full prescribing information for the recommended titration and maximum recommended dosage. (2.2)
- Take at least 1 hour before a meal or at least 2 hours after a meal. Do not open capsules. (2.2)
- Geriatric patients: Recommended starting dosage of COBENFY is 50 mg/20 mg orally twice daily. Consider a slower titration. The maximum recommended dosage is 100 mg/20 mg twice daily. (2.3)
DOSAGE FORMS AND STRENGTHS
Capsules (xanomeline/trospium chloride): 50 mg/20 mg, 100 mg/20 mg, 125 mg/30 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of Urinary Retention: COBENFY can cause urinary retention. Geriatric patients and patients with bladder outlet obstruction and incomplete bladder emptying are at increased risk. Monitor patients for symptoms of acute urinary retention. (5.1)
- Risk of Use in Patients with Hepatic Impairment: COBENFY is contraindicated in patients with moderate to severe hepatic impairment and is not recommended in patients with mild hepatic impairment. (5.2)
- Risk of Use in Patients with Biliary Disease: Assess liver enzymes and bilirubin prior to initiating COBENFY and as clinically indicated. Discontinue COBENFY in the presence of signs or symptoms of substantial liver injury. (5.3)
- Decreased Gastrointestinal Motility: COBENFY may decrease gastrointestinal motility. Use with caution in patients with gastrointestinal obstructive disorders because of the risk of gastric retention. (5.4)
- Risk of Angioedema: Angioedema of the face, lips, tongue and/or larynx has been reported with COBENFY. (5.5)
- Risk of Use in Patients with Narrow-angle Glaucoma: Use COBENFY only if the potential benefits outweigh the risks and with careful monitoring. (5.6)
- Increases in Heart Rate: COBENFY may increase heart rate. Assess heart rate at baseline and as clinically indicated during treatment with COBENFY. (5.7)
- Anticholinergic Adverse Reactions in Patients with Renal Impairment: COBENFY is not recommended for use in patients with moderate and severe renal impairment. Anticholinergic adverse reactions are expected to be greater in these patients. (5.8)
- Central Nervous System Effects: COBENFY may be associated with CNS effects. Advise patients not drive or operate heavy machinery until they know how COBENFY affects them. (5.9)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and at least twice placebo) were nausea, dyspepsia, constipation, vomiting, hypertension, abdominal pain, diarrhea, tachycardia, dizziness, and gastrointestinal reflux disease. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs Eliminated by Active Tubular Secretion: Monitor for increased frequency and/or severity of adverse reactions related to COBENFY and to drugs eliminated by active tubular secretion. (7.1)
- Strong CYP2D6 Inhibitors: Monitor for increased frequency and/or severity of COBENFY-related adverse reactions. (7.1)
- Sensitive Substrates of CYP3A4 or P-glycoprotein: Monitor for increased frequency and/or severity of adverse reactions from these substrates. (7.1)
- Antimuscarinic Drugs: Monitor for increased frequency or severity of anticholinergic adverse reactions. (7.2)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing and Monitoring Prior to Initiation and During Treatment with COBENFY
2.2 Recommended Dosage and Administration
2.3 Dosage Recommendations in Geriatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
5.2 Risk of Use in Patients with Hepatic Impairment
5.3 Risk of Use in Patients with Biliary Disease
5.4 Decreased Gastrointestinal Motility
5.5 Risk of Angioedema
5.6 Risk of Use in Patients with Narrow-angle Glaucoma
5.7 Increases in Heart Rate
5.8 Anticholinergic Adverse Reactions in Patients with Renal Impairment
5.9 Central Nervous System Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Clinically Significant Drug Interactions with COBENFY
7.2 Other Antimuscarinic Drugs
7.3 Effects on Absorption of Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing and Monitoring Prior to Initiation and During Treatment with COBENFY
- Assess liver enzymes and bilirubin prior to initiating COBENFY and as clinically indicated during treatment [see Contraindications (4) and Warnings and Precautions (5.2, 5.3)].
- Assess heart rate at baseline and as clinically indicated during treatment [see Warnings and Precautions (5.7)].
2.2 Recommended Dosage and Administration
The recommended dosage of COBENFY is as follows:
- The recommended starting dosage is one 50 mg/20 mg capsule (contains 50 mg of xanomeline and 20 mg of trospium chloride) orally twice daily for at least two days.
- Increase the dosage to one 100 mg/20 mg capsule (contains 100 mg of xanomeline and 20 mg of trospium chloride) orally twice daily for at least five days.
- The dosage may be increased to one 125 mg/30 mg capsule (contains 125 mg of xanomeline and 30 mg of trospium chloride) orally twice daily based on patient tolerability and response [see Clinical Studies (14)].
- Maximum recommended dosage is 125 mg/30 mg orally twice daily.
Administer COBENFY orally at least one hour before a meal or at least two hours after a meal [see Clinical Pharmacology (12.3)]. Do not open the capsules.
2.3 Dosage Recommendations in Geriatric Patients
The recommended starting dosage of COBENFY in geriatric patients is one 50 mg/20 mg capsule orally twice daily. Consider a slower titration for geriatric patients. The maximum recommended dosage in geriatric patients is one 100 mg/20 mg capsule twice daily [see Warnings and Precautions (5.1, 5.8) and Use in Specific Populations (8.5)].
-
3 DOSAGE FORMS AND STRENGTHS
COBENFY is available as:
- 50 mg/20 mg (xanomeline/trospium chloride): Buff capsules imprinted with Karuna 50/20 mg
- 100 mg/20 mg (xanomeline/trospium chloride): Brown capsules imprinted with Karuna 100/20 mg
- 125 mg/30 mg (xanomeline/trospium chloride): Swedish Orange capsules imprinted with Karuna 125/30 mg
-
4 CONTRAINDICATIONS
COBENFY is contraindicated in patients with:
- urinary retention [see Warnings and Precautions (5.1)].
- moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment [see Warnings and Precautions (5.2)].
- gastric retention [see Warnings and Precautions (5.4)].
- history of hypersensitivity to COBENFY or trospium chloride. Angioedema has been reported with COBENFY and trospium chloride [see Warnings and Precautions (5.5)].
- untreated narrow-angle glaucoma [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
COBENFY can cause urinary retention [see Adverse Reactions (6.1)]. Geriatric patients and patients with clinically significant bladder outlet obstruction and incomplete bladder emptying (e.g., patients with benign prostatic hyperplasia (BPH), diabetic cystopathy) may be at increased risk of urinary retention [see Use in Specific Populations (8.5)].
COBENFY is contraindicated in patients with pre-existing urinary retention [see Contraindications (4)] and is not recommended in patients with moderate or severe renal impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
In patients taking COBENFY, monitor for symptoms of urinary retention, including urinary hesitancy, weak stream, incomplete bladder emptying, and dysuria. Instruct patients to be aware of the risk and promptly report symptoms of urinary retention to their healthcare provider. Urinary retention is a known risk factor for urinary tract infections. In patients with symptoms of urinary retention, consider reducing the dose of COBENFY, discontinuing COBENFY, or referring patients for urologic evaluation as clinically indicated.
5.2 Risk of Use in Patients with Hepatic Impairment
Patients with hepatic impairment have higher systemic exposures of xanomeline, a component of COBENFY, compared to patients with normal hepatic function, which may result in increased incidence of COBENFY-related adverse reactions [see Clinical Pharmacology (12.3)].
COBENFY is contraindicated in patients with moderate or severe hepatic impairment [see Contraindications (4)]. COBENFY is not recommended in patients with mild hepatic impairment [see Use in Specific Populations (8.7)andClinical Pharmacology (12.3)].
Assess liver enzymes prior to initiating COBENFY and as clinically indicated during treatment.
5.3 Risk of Use in Patients with Biliary Disease
In clinical studies with COBENFY, transient increases in liver enzymes with rapid decline occurred, consistent with transient biliary obstruction due to biliary contraction and possible gallstone passage [see Adverse Reactions (6.1)].
COBENFY is not recommended for patients with active biliary disease such as symptomatic gallstones. Assess liver enzymes and bilirubin prior to initiating COBENFY and as clinically indicated during treatment. The occurrence of symptoms such as dyspepsia, nausea, vomiting, or upper abdominal pain should prompt assessment for gallbladder disorders, biliary disorders, and pancreatitis, as clinically indicated.
Discontinue COBENFY in the presence of signs or symptoms of substantial liver injury such as jaundice, pruritus, or alanine aminotransferase levels more than five times the upper limit of normal or five times baseline values.
5.4 Decreased Gastrointestinal Motility
COBENFY contains trospium chloride. Trospium chloride, like other antimuscarinic agents, may decrease gastrointestinal motility. Administer COBENFY with caution in patients with gastrointestinal obstructive disorders because of the risk of gastric retention [see Contraindications (4)]. Use COBENFY with caution in patients with conditions such as ulcerative colitis, intestinal atony, and myasthenia gravis.
5.5 Risk of Angioedema
Angioedema of the face, lips, tongue, and/or larynx has been reported with COBENFY and trospium chloride, a component of COBENFY [see Adverse Reactions (6.2)]. In one case, angioedema occurred after the first dose of trospium chloride. Angioedema associated with upper airway swelling may be life-threatening. If involvement of the tongue, hypopharynx, or larynx occurs, discontinue COBENFY and initiate appropriate therapy and/or measures necessary to ensure a patent airway. COBENFY is contraindicated in patients with a history of hypersensitivity to trospium chloride.
5.6 Risk of Use in Patients with Narrow-angle Glaucoma
Pupillary dilation may occur due to the anticholinergic effects of COBENFY. This may trigger an acute angle closure attack in patients with anatomically narrow angles. In patients known to have anatomically narrow angles, COBENFY should only be used if the potential benefits outweigh the risks and with careful monitoring [see Contraindications (4)].
5.7 Increases in Heart Rate
COBENFY can increase heart rate [see Adverse Reactions (6.1)]. Assess heart rate at baseline and as clinically indicated during treatment with COBENFY [see Dosage and Administration (2.1)].
5.8 Anticholinergic Adverse Reactions in Patients with Renal Impairment
Trospium chloride, a component of COBENFY, is substantially excreted by the kidney. COBENFY is not recommended in patients with moderate or severe renal impairment (estimated glomerular filtration rate (eGFR) <60 mL/min). Systemic exposure of trospium chloride is higher in patients with moderate and severe renal impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Therefore, anticholinergic adverse reactions (including dry mouth, constipation, dyspepsia, urinary tract infection, and urinary retention) are expected to be greater in patients with moderate and severe renal impairment.
5.9 Central Nervous System Effects
Trospium chloride, a component of COBENFY, is associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions (6.1)]. A variety of CNS anticholinergic effects have been reported with trospium chloride, including dizziness, confusion, hallucinations, and somnolence. Monitor patients for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how COBENFY affects them. If a patient experiences anticholinergic CNS effects, consider dose reduction or drug discontinuation.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Urinary Retention [see Warnings and Precautions (5.1)]
- Risk of Use in Patients with Hepatic Impairment [see Warnings and Precautions (5.2)]
- Risk of Use in Patients with Biliary Disease [see Warnings and Precautions (5.3)]
- Decreased Gastrointestinal Motility [see Warnings and Precautions (5.4)]
- Risk of Angioedema [see Warnings and Precautions (5.5)]
- Risk of Use in Patients with Narrow-angle Glaucoma [see Warnings and Precautions (5.6)]
- Increases in Heart Rate [see Warnings and Precautions (5.7)]
- Anticholinergic Adverse Reactions in Patients with Renal Impairment [see Warnings and Precautions (5.8)]
- Central Nervous System Effects [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
COBENFY was evaluated for safety in a total of 1,594 subjects exposed to one or more doses, including 1,135 adult patients with schizophrenia and 389 healthy subjects. A total of 347 COBENFY-treated patients had at least 6 months of exposure and 150 patients had at least 1 year of exposure (defined as ≥ 50 weeks) from open-label studies.
The adverse reaction findings are based on two pooled 5-week, placebo-controlled, flexible-dose studies in 504 adult patients with schizophrenia in which COBENFY or placebo was started at an initial dose of 50 mg/20 mg twice daily for the first 2 days followed by 100 mg/20 mg twice daily for the remainder of Week 1 (Days 3 to 7). On Day 8, dosing was titrated to 125 mg/30 mg twice daily unless the patient could not tolerate it. All patients had the option to return to 100 mg/20 mg twice daily for the remainder of the treatment period [see Clinical Studies (14)].
In the 5-week placebo-controlled studies, 6% of patients treated with COBENFY and 4% of placebo-treated patients discontinued participation due to adverse reactions. Adverse reactions that led to study discontinuation in ≥1% of patients treated with COBENFY include nausea (2%) and vomiting (1%).
The most common adverse reactions (≥5% and at least twice placebo) were nausea, dyspepsia, constipation, vomiting, hypertension, abdominal pain, diarrhea, tachycardia, dizziness, and gastroesophageal reflux disease.
Adverse reactions reported with COBENFY at an incidence of at least 2% in patients exposed to COBENFY and greater than the rate of placebo are shown in Table 1.
Table 1: Adverse Reactions Reported in ≥2% of COBENFY-Treated Patients and Greater than Rate of Placebo in Two 5-week Schizophrenia Trials a Dyspepsia includes dyspepsia, esophageal discomfort b Hypertension includes hypertension, blood pressure increased, labile hypertension, orthostatic hypertension c Abdominal Pain includes abdominal discomfort, abdominal pain upper, abdominal pain, abdominal pain lower, abdominal tenderness d Tachycardia includes tachycardia, heart rate increased, sinus tachycardia e Cough: includes cough, productive cough f EPS (non-akathisia) includes dyskinesia, drooling, dystonia, extrapyramidal disorder, muscle contraction involuntary, muscle spasms COBENFY
(N=251)
Placebo
(N=253)
Nausea
19%
4%
Dyspepsiaa
18%
5%
Constipation
17%
7%
Vomiting
15%
1%
Hypertensionb
11%
2%
Abdominal Painc
8%
4%
Diarrhea
6%
2%
Tachycardiad
5%
2%
Dizziness
5%
2%
Gastroesophageal reflux disease
5%
<1%
Dry mouth
4%
2%
Somnolence
3%
2%
Vision blurred
3%
0%
Salivary hypersecretion
2%
0%
Orthostatic hypotension
2%
1%
Coughe
2%
1%
Extrapyramidal symptoms (EPS), non-akathisiaf
2%
<1%
Increases in Heart Rate
In a dedicated 8-week clinical study, 24-hour ambulatory blood pressure monitoring (ABPM) was conducted in 133 patients with schizophrenia. A total of 95 patients had acceptable ABPM recordings at both baseline and Week 8. In that group, there was a mean change in 24-hour heart rate of 9.8 beats per minute (bpm) (95% CI 7.5, 12.2) from baseline to Week 8.In the two placebo-controlled schizophrenia studies, COBENFY was associated with increases in heart rate compared to placebo, with peak elevation occurring on Day 8 of study treatment (13.5 bpm in the COBENFY group and 4.0 bpm in the placebo group), partially attenuating with continued dosing (11.4 bpm in the COBENFY group and 5.5 bpm in the placebo group at Week 5).
Liver Enzyme Elevations
In the 5-week, placebo-controlled schizophrenia studies, the proportions of patients with ALT or AST elevations of ≥3 times the upper limits of the normal reference range were 2.8% (6/214) for COBENFY-treated patients compared to 0.4% (1/224) of placebo-treated patients. Twenty-five (1.6%) of the total 1,594 subjects exposed to COBENFY had elevated liver enzymes. The majority of liver enzyme elevations occurred within the first month of treatment and resolved with continued COBENFY use, suggestive of liver adaptation; some cases required treatment interruption, and one was associated with an increase in bilirubin.Urinary Retention
In the 5-week, placebo-controlled studies, urinary retention (urinary hesitation, dysuria, and urinary retention) was reported in 0.8% of COBENFY-treated patients and 0.4% on placebo. In the long-term, open-label studies, urinary retention was reported in 3.5% of COBENFY-treated patients. Urinary retention was more common in males, geriatric patients, and those with certain risk factors [see Warnings and Precautions (5.1)]. Urinary retention occurred at all doses but was predominately observed at the maximum COBENFY dose. In the long-term, open-label studies, urinary tract infections were reported in 2.3% of COBENFY-treated patients and were more commonly reported in females than males. Of the total 1,594 subjects exposed to COBENFY (including healthy volunteers and patients with schizophrenia or other conditions), four subjects required a Foley catheter, including one with elevated serum creatinine and one with urinary tract infections. Four subjects with urinary retention required reduction of COBENFY dose, four discontinued COBENFY, and four received medications for the treatment of benign prostatic hyperplasia (BPH).6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of trospium chloride, one of the components of COBENFY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular – chest pain, hypertensive crisis, palpitations, supraventricular tachycardia, syncope
- Gastrointestinal – gastritis
- General – rash
- Musculoskeletal – rhabdomyolysis
- Nervous System – confusion, delirium, dizziness, hallucinations, somnolence, vision abnormal
- Skin and subcutaneous tissue disorders – angioedema, anaphylactic reaction, Stevens-Johnson syndrome
-
7 DRUG INTERACTIONS
7.1 Clinically Significant Drug Interactions with COBENFY
Table 2 displays clinically significant drug interactions with COBENFY.
Table 2: Clinically Significant Drug Interactions with COBENFY Strong Inhibitors of CYP2D6
Clinical Implication:
CYP2D6 contributes significantly to the metabolism of xanomeline, a component of COBENFY. Concomitant use of COBENFY with strong CYP2D6 inhibitors may increase plasma concentrations of xanomeline, which may increase the frequency and/or severity of adverse reactions from COBENFY [see Clinical Pharmacology (12.3)].
Prevention or Management:
Monitor patients for increased frequency and/or severity of adverse reactions related to COBENFY in patients taking COBENFY with strong inhibitors of CYP2D6.
Drugs Eliminated by Active Tubular Secretion
Clinical Implication:
Concomitant use of COBENFY with drugs that are eliminated by active tubular secretion may increase plasma concentrations of trospium a component of COBENFY, and/or the concomitantly used drug due to competition for this elimination pathway, which may increase the frequency and/or severity of adverse reactions from COBENFY or the drug eliminated by active tubular secretion [see Clinical Pharmacology (12.3)].
Prevention or Management:
Monitor patients for increased frequency and/or severity of adverse reactions related to COBENFY and adverse reactions related to drugs eliminated by active tubular secretion in patients concomitantly receiving such drugs.
Oral Drugs That Are Sensitive Substrates of CYP3A4
Clinical Implication:
Xanomeline, a component of COBENFY, transiently inhibits CYP3A4 locally in the gut but not systemically. Concomitant use of COBENFY with oral drugs that are sensitive substrates of CYP3A4 may result in increased plasma concentrations of the oral drugs that are sensitive substrates of CYP3A4. This may increase the frequency and/or severity of adverse reactions from such substrates [see Clinical Pharmacology (12.3)].
Prevention or Management:
Monitor patients for increased frequency and/or severity of adverse reactions related to oral drugs that are sensitive substrates of CYP3A4 in patients taking COBENFY with such substrates.
Oral Drugs That Are Substrates of P-glycoprotein
Clinical Implication:
Xanomeline, a component of COBENFY, transiently inhibits P-glycoprotein locally in the gut but not systemically. Concomitant use of COBENFY with oral drugs that are substrates of P-glycoprotein may result in increased plasma concentrations of the oral drugs that are substrates of P-glycoprotein, which may increase the frequency and/or severity of adverse reactions from such substrates [see Clinical Pharmacology (12.3)].
Prevention or Management:
Monitor patients for increased frequency and/or severity of adverse reactions related to oral drugs that are narrow therapeutic index substrates of P-glycoprotein in patients taking COBENFY with such substrates.
7.2 Other Antimuscarinic Drugs
Concomitant use of COBENFY with other antimuscarinic drugs that produce anticholinergic adverse reactions (e.g., dry mouth, constipation) may increase the frequency and/or severity of such effects. Monitor patients for increased frequency and/or severity of anticholinergic adverse reactions when COBENFY is used concomitantly with other antimuscarinic drugs.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors outcomes in women exposed to psychiatric medications, including COBENFY, during pregnancy. Healthcare providers are encouraged to advise patients to register by calling 1-866-961-2388 or visiting online at https://womensmentalhealth.org/research/pregnancyregistry/atypicalantipsychotic/.Risk Summary
There are no available data on COBENFY use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are risks to the mother associated with untreated schizophrenia (see Clinical Considerations). In animal reproduction studies, oral administration of xanomeline alone or in combination with trospium chloride during the period of organogenesis or during pregnancy and lactation caused maternal toxicities of adverse clinical signs, decreased body weight, weight gain and food consumption, and/or maternal death. At these maternally toxic doses, embryofetal and developmental toxicities included decreased fetal and neonatal weight, stillborn pups, and/or neonatal deaths. The no observed adverse effect level (NOAEL) of xanomeline or xanomeline/trospium chloride combination for maternal, embryofetal, and/or developmental toxicity is equal to or higher than the xanomeline and trospium chloride dose at the maximum recommended human dose (MRHD) of 250/60 mg xanomeline/trospium chloride, based on mg/m2 body surface area (BSA) (see Data).The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
There is a risk to the pregnant patient from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide. Schizophrenia is associated with adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.Data
Animal Data
Pregnant rats were orally treated during the period of organogenesis with 150 mg/kg/day xanomeline alone, 100 mg/kg/day trospium chloride alone, or xanomeline/trospium chloride combination at 30/25, 75/50, and 150/100 mg/kg/day, respectively. Xanomeline alone and the high dose combination caused maternal toxicities of decreased body weight, weight gain, and food consumption. At these maternally toxic doses, fetal weights were decreased. The NOAEL for maternal and embryofetal toxicity is 75/50 mg/kg/day for the combination, which is approximately 3 and 8 times the xanomeline and trospium chloride dose, respectively, at the MRHD of 250/60 mg xanomeline/trospium chloride, based on BSA. No fetal malformation was observed. Trospium chloride alone did not cause maternal or embryofetal toxicity.Pregnant rabbits were orally treated during the period of organogenesis with120 mg/kg/day xanomeline alone, 80 mg/kg/day trospium chloride alone, or xanomeline/trospium chloride combination at 30/20, 60/40, and 120/80 mg/kg/day, respectively. Xanomeline alone and the high dose combination caused maternal toxicities of decreased body weight, weight gain, and food consumption, and/or early abortion. At these maternally toxic doses, decreased fetal weight and decreased fetal viability (increased resorption and post-implantation loss) were observed. The NOAEL for maternal and embryofetal toxicity is 60/40 mg/kg/day for the xanomeline/trospium chloride combination, which is 5 and 13 times the xanomeline and trospium chloride dose, respectively at the MRHD, based on BSA. No fetal malformation was observed. Trospium chloride alone did not cause maternal or embryofetal toxicity.
Rats were orally treated during pregnancy and lactation with 30, 75, and 150 mg/kg/day xanomeline alone, 100 mg/kg/day trospium chloride alone, or xanomeline/trospium chloride combination at 30/25, 75/50, and 150/100 mg/kg/day, respectively. Xanomeline alone at ≥ 75 mg/kg/day or in combination with trospium chloride at ≥ 75/50 mg/kg/day caused maternal toxicity of adverse clinical signs, decreased body weight, weight gain, food consumption, and maternal death. At these maternally toxic doses, developmental toxicity was observed in the offspring, including growth suppression (decreased body weight and weight gain), delayed developmental landmarks, stillborn pups, and neonatal deaths. No drug effect was observed on the neurobehavioral function, including learning and memory, or the reproductive capacity of the offspring. The NOAEL for maternal and developmental toxicity is 30/25 mg/kg/day for the xanomeline/trospium chloride combination, which is approximately 1 and 4 times the xanomeline and trospium chloride dose, respectively at the MRHD, based on BSA. Trospium chloride alone did not cause maternal or developmental toxicity.
Pregnant rats were treated during the period of organogenesis with trospium chloride at doses up to 200 mg/kg/day. No malformation or fetal toxicity was observed up to 200 mg/kg/day, which is approximately 32 times the trospium chloride dose at the MRHD of 250/60 mg xanomeline/trospium chloride based on BSA.
Pregnant rabbits were treated during the period of organogenesis with trospium chloride at doses up to 200 mg/kg/day. Maternal toxicity (reduced feces, hunched posture, and diarrhea) was observed at 200 mg/kg/day. The NOAEL for maternal toxicity is 20 mg/kg/day, which is approximately 3 times the trospium chloride dose at the MRHD based on BSA.
Rats were orally treated during pregnancy and lactation with trospium chloride at doses up to 200 mg/kg/day. Maternal toxicity (death, irregular breathing, increased excitability) and neonatal deaths were observed at 200 mg/kg/day, which is approximately 32 times the MRHD, based on BSA. The NOAEL for maternal and developmental toxicity is 20 mg/kg/day, which is approximately 3 times the trospium chloride dose at the MRHD, based BSA.
8.2 Lactation
Risk Summary
There are no data on the presence of xanomeline or trospium in human milk, the effects on the breastfed infant, or the effects on milk production. Xanomeline and trospium are present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COBENFY and any potential adverse effects on the breastfed infant from COBENFY or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of COBENFY in pediatric patients have not been established.
8.5 Geriatric Use
Controlled clinical studies of COBENFY did not include patients older than 65 years of age to determine whether they respond differently from younger adult patients.
Because COBENFY can increase the risk of urinary retention in geriatric patients, including older males with bladder outlet obstruction due to benign prostatic hyperplasia (BPH), a slower titration and lower maximum dosage is recommended in geriatric patients [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
8.6 Renal Impairment
Patients with mild renal impairment (eGFR 60 to <90 mL/min) showed higher systemic exposures to trospium chloride and xanomeline, the components of COBENFY, compared to subjects with normal renal function. However, in the adequate and well-controlled clinical studies, the safety profiles in patients with mild renal impairment were similar to those observed in patients with normal renal function (eGFR ≥90 mL/min). Therefore, the recommended dosage in patients with mild renal impairment is the same as the recommended dosage for patients with normal renal function.
Use of COBENFY is not recommended in patients with moderate or severe renal impairment (eGFR<60 mL/min) [seeWarnings and Precautions (5.1, 5.8) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with mild to moderate hepatic impairment (Child-Pugh Class A and B, respectively) have higher xanomeline exposures compared to patients with normal hepatic function [see Clinical Pharmacology (12.3)]. The pharmacokinetics of COBENFY were not studied in patients with severe hepatic impairment (Child-Pugh Class C).
Use of COBENFY is contraindicated in patients moderate or severe hepatic impairment [see Contraindications (4) and Warnings and Precautions (5.2)]. It is not recommended in patients with mild hepatic impairment.
-
10 OVERDOSAGE
Overdose of COBENFY may produce cholinergic, anticholinergic or a combination of cholinergic and anticholinergic signs and symptoms:
- Cholinergic Signs and Symptoms: seizures, vomiting, diarrhea, abdominal pain, hyperhidrosis, salivary hypersecretion, and hypotension possibly preceded by hypertension.
- Anticholinergic Signs and Symptoms (geriatric patients may be more susceptible): delirium, agitation, garbled speech, dizziness, hypertension, tachycardia, dry mouth and eyes, ileus, blurred vision, and urinary retention.
Consider calling the Poison Help Line 1-800-222-1222 or a medical toxicologist for specific treatment recommendations.
-
11 DESCRIPTION
COBENFY is a combination of xanomeline, a muscarinic agonist, and trospium chloride, a muscarinic antagonist.
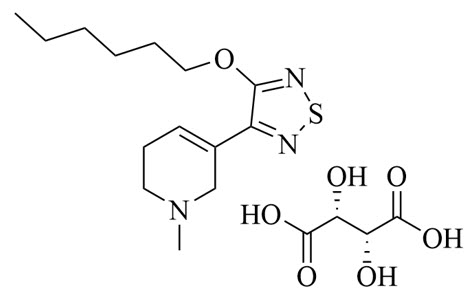
The chemical name of xanomeline tartrate is pyridine, 3-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1,2,5,6-tetrahydro-1-methyl-, (2R,3R)-2,3-dihydroxybutanedioate (1:1). Its molecular formula is C14H23N3OS.C4H6O6 and its molecular weight is 431.51 g/mol. Xanomeline tartrate is a white to slightly tan crystalline solid. Xanomeline tartrate is highly soluble in protic solvents, such as methanol and water, and in polar organic solvents such as DMF and dimethyl sulfoxide (DMSO). It is poorly soluble in lipophilic organic solvents, such as hexane or octanol.
The chemical structure of xanomeline tartrate is:
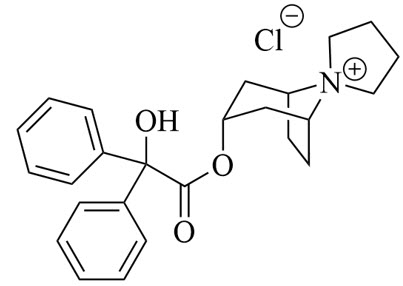
Trospium chloride is a quaternary ammonium compound with the chemical name of spiro[8-azoniabicyclo[3.2.1]octane-8,1′-pyrrolidinium], 3-[(2-hydroxy-2,2-diphenylacetyl)oxy]-, chloride (1:1), (1α,3β,5α). The molecular formula of trospium chloride is C25H30NO3.Cl and its molecular weight is 427.96 g/mol. Trospium chloride is a fine, colorless to slightly yellow, crystalline solid. Trospium chloride is highly soluble in water, freely soluble in methanol, and practically insoluble in methylene chloride.
The chemical structure of trospium chloride is:
COBENFY (xanomeline and trospium chloride) is for oral administration and is available in capsules in the following strengths:
- 50 mg/20 mg (equivalent to 76.7 mg xanomeline tartrate and 18.3 mg trospium).
- 100 mg/20 mg (equivalent to 153.3 mg xanomeline tartrate and 18.3 mg trospium).
- 125 mg/30 mg (equivalent to 191.7 mg xanomeline tartrate and 27.5 mg trospium).
COBENFY capsules contain a combination of pellets of xanomeline and pellets of trospium chloride.
Inactive ingredients: The xanomeline tartrate pellets contain ascorbic acid, microcrystalline cellulose, and talc.
The trospium chloride pellets contain lactose monohydrate, microcrystalline cellulose, and talc.
The capsules, printed with black ink, contain black iron oxide (only 100 mg/20 mg), hypromellose, red iron oxide, titanium dioxide, and yellow iron oxide (only 50 mg/20 mg and 100 mg/20 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of xanomeline in the treatment of schizophrenia is unclear; however, its efficacy is thought to be due to its agonist activity at M1 and M4 muscarinic acetylcholine receptors in the central nervous system.
Trospium chloride is a muscarinic antagonist. Trospium chloride antagonizes the muscarinic receptors primarily in the peripheral tissues.
12.2 Pharmacodynamics
Xanomeline binds to muscarinic receptors M1 to M5 with comparable affinity (Ki=10, 12, 17, 7, and 22 nM for the M1, M2, M3, M4, and M5 receptors, respectively) and exhibits higher agonist activity at the M1 and M4 receptors.
Trospium chloride antagonizes the muscarinic receptors primarily in peripheral tissues.
Cardiac Electrophysiology
At the maximum recommended dosage of 125 mg/30 mg twice daily, COBENFY does not prolong the QT interval to any clinically relevant extent.12.3 Pharmacokinetics
Following COBENFY administration, xanomeline area under the plasma concentration-time curve during a 12-hour dosing interval (AUC0-12) at steady state and maximum concentration (Cmax) increased 50% when the COBENFY dose increased from 100 mg/20 mg twice daily to 125 mg/30 mg twice daily. Trospium exposures increase dose-proportionally over the COBENFY dosage range of 100 mg/20 mg twice daily to 125 mg/30 mg twice daily.
Pharmacokinetic properties of COBENFY are provided in Table 3.
Table 3: Pharmacokinetic Properties of COBENFY Parameter
Xanomeline
Trospium
General Information
Dose proportionality
Greater than proportional
Proportional
Accumulationa
2 to 3-fold
2 to 3-fold
Time to steady state
3 to 5 days
3 to 5 days
Absorption
Tmax
2 hours
1 hour
Effect of food: PK in fed state (compared to fasted state)
High fat mealb
Cmax
Unchanged
Reduced 70% to 75%
AUC
Increased 30%
Reduced 85% to 90%
Low fat mealb
Cmax
Unchanged
Reduced 70% to 75%
AUC
Unchanged
Reduced 85% to 90%
Distribution
Central volume of distribution (oral)
10,800 Liters
531 Liters
Plasma protein binding
Approximately 95%
Approximately 80%
Elimination
Half-life (t1/2)
5 hours
6 hours
Apparent clearance
1950 Liters/hour
796 Liters/hour
Renal clearance
0.085 Liters/hour
21 Liters/hour
Metabolism
Primary metabolic pathways
CYP450
2D6, 2B6, 1A2, 2C9, and 2C19
Unlikely
Other
Flavin monooxygenases (FMO1 and FMO3)
Ester hydrolysis and glucuronic acid conjugation
(not fully characterized)
Excretion
Urine
Total
78%
Unknown
Unchanged
Less than 0.01%
85-90%
Tubular secretion
Unknown
Yes
Feces
Total
12%
Unknown
Unchanged
Unknown
Unknown
Abbreviations: AUC = Area under the time-concentration curve; Cmax = Maximum concentration; Tmax =Time to Cmax
a Dose-normalized accumulation at steady state
b High-fat high-calorie meal is 800-1000 calories, 50% from fat; a low-fat meal is 400-500 calories, 25% from fat
Specific Populations
Geriatric Patients
Population pharmacokinetic analysis suggests that AUC0-12h and Cmax of trospium at steady state were 60% higher and 36% higher, respectively, in subjects 65 years and older compared to subjects younger than 65 years old. The exposures (AUC0-12h and Cmax) of xanomeline at steady state were not different between subjects 65 years and older and subjects younger than 65 years old [see Dosage and Administration (2.3) and Use in Specific Populations (8.5)].Male and Female Patients
Plasma concentrations of xanomeline and trospium are similar between females and males.Racial or Ethnic Groups
Most subjects in clinical studies were Black.
Xanomeline and trospium exposure did not differ between Black and non-Black subjects. Studies have included too few subjects of Asian descent to evaluate comparisons.Patients with Renal Impairment
The effect of renal impairment on xanomeline and trospium exposure was assessed in a dedicated study that enrolled healthy subjects and subjects with mild, moderate, or severe renal impairment. Estimated glomerular filtration rate (eGFR) was determined by the MDRD equation.Plasma concentrations of xanomeline and trospium increased with increasing renal dysfunction [see Use in Specific Populations (8.6)]. For xanomeline, compared to subjects with normal renal function (eGFR: ≥90 mL/min), the steady-state Cmax and AUC0-12h were 2.1 and 1.9 times higher in subjects with mild renal impairment (eGFR: 60 to <90 mL/min), 2.4 and 2.1 times higher in subjects with moderate renal impairment (eGFR: 30 to <60 mL/min), and 2.6 and 2.4 times higher in subjects with severe renal impairment (eGFR: <30 mL/min). For trospium, compared to subjects with normal renal function, the steady-state Cmax and AUC0-12h were 1.6 and 1.6 times higher in subjects with mild renal impairment, 2.7 and 2.2 times higher in subjects with moderate renal impairment, and 2.9 and 2.9 times higher in subjects with severe renal impairment.
Patients with Hepatic Impairment
The effect of hepatic impairment on xanomeline and trospium in combination was assessed in a dedicated study that enrolled healthy subjects and subjects with mild or moderate hepatic impairment as determined by their Child-Pugh score.Plasma concentrations of xanomeline increased with increasing hepatic dysfunction [see Use in Specific Populations (8.7)]. In subjects with mild hepatic impairment (Child-Pugh Class A), the steady-state Cmax and AUC0-12h of xanomeline was 2.8 and 2.6 times that in subjects with normal hepatic function. Mild and moderate hepatic impairment did not substantially affect trospium exposure, but significantly impacted xanomeline exposures. In subjects with moderate hepatic impairment (Child-Pugh Class B), the steady-state Cmax and AUC0-12h of xanomeline was at least 7 times that in subjects with normal hepatic function [see Contraindications (4) and Warnings and Precautions (5.2)].
The effect of severe hepatic impairment on xanomeline and trospium exposure was not evaluated.
Body Weight
Compared to subjects weighing 70 kg, xanomeline exposures were 30 to 35% lower and trospium exposures were 20 to 35% lower in subjects weighing 120 kg. The lower exposures observed in subjects weighing 120 kg are expected to be clinically not meaningful.Drug Interaction Studies
Drugs Eliminated by Active Tubular SecretionActive tubular excretion is a major elimination pathway for trospium. Trospium has the potential for pharmacokinetic interactions with other drugs that are eliminated by active tubular secretion. Coadministration of COBENFY with these drugs may increase plasma concentrations of trospium and/or the coadministered drug due to competition for this elimination pathway [see Drug Interactions (7.1)].
Metformin
A drug interaction study was conducted in which extended-release trospium chloride 60 mg once daily was coadministered with metformin hydrochloride 500 mg twice daily under steady-state conditions in 44 healthy subjects. Co-administration of 500 mg metformin immediate-release tablets twice daily reduced the steady-state systemic exposure of trospium by approximately 29% for mean AUC0-24 and by 34% for mean Cmax. The steady-state pharmacokinetics of metformin were comparable when administered with or without 60 mg extended-release trospium chloride once daily under fasted conditions. The effect of metformin at higher doses on trospium pharmacokinetics is unknown.Drugs That Inhibit CYP2D6
CYP2D6 is a significant contributor to the metabolism of xanomeline. Drugs that are inhibitors of CYP2D6 may increase xanomeline concentrations in plasma [see Drug Interactions (7.1)].Drugs That Are Substrates of P-glycoprotein
In vitro data suggest that xanomeline does not inhibit P-glycoprotein systemically, but it may transiently inhibit P-glycoprotein locally in the intestine after dosing. COBENFY may increase plasma concentrations of coadministered P-gp substrates [see Drug Interactions (7.1)].Drugs That Are Substrates of CYP3A4
In vitro data suggest that xanomeline does not inhibit CYP3A4 systemically, but it may transiently inhibit CYP3A4 locally in the intestine after dosing. COBENFY may increase plasma concentrations of coadministered CYP3A4 substrates [see Drug Interactions (7.1)].12.5 Pharmacogenomics
CYP2D6 is a significant contributor to the metabolism of xanomeline. The gene encoding CYP2D6 has polymorphisms that impact protein function. Based on a population pharmacokinetic analysis, compared to subjects with normal CYP2D6 function, the median Cmax and median AUC0-12h of xanomeline were estimated to increase by 28% and 15% in CYP2D6 intermediate metabolizers (N=84) and decrease by 43% in both parameters for ultrarapid metabolizers (N=12). The pharmacokinetics of xanomeline have not been adequately characterized in subjects who are poor metabolizers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Xanomeline
Xanomeline was administered to rats in the diet at doses of 9, 37, and 134 mg/kg/day in males and 11, 46, and 170 mg/kg/day in females, respectively, for two years. Biliary hyperplasia was observed in all groups with increased incidence and/or severity at ≥ 37 and 46 mg/kg/day in males and females, respectively, relative to controls. There was no increase in the incidence of tumors at doses up to 37 and 46/mg/kg/day in males and females, respectively; these doses are 1.4 to 1.8 times higher than the xanomeline dose at the MRHD of 250/60 mg xanomeline/trospium, based on mg/m2 BSA. The high doses of 134 and 170 mg/kg/day in males and females exceeded the maximum tolerated dose (MTD), precluding an adequate assessment for carcinogenic effect at this dose.Xanomeline was administered to mice in the diet at doses of 52, 174, and 559 mg/kg/day for 21 months in both sexes. Xanomeline did not increase the incidence of tumors in mice at doses up to 174 mg/kg/day, which is approximately 3 times the xanomeline dose at the MRHD, based on BSA. The high dose of 559 mg/kg/day exceeded the MTD, precluding an adequate assessment for carcinogenic effect at this dose.
Trospium chloride
Trospium chloride did not increase the incidence of tumors in rats treated for 104 weeks at doses up to 200 mg/kg/day, which is approximately 32 times the trospium chloride dose at the MRHD, based on BSA.Trospium chloride did not increase the incidence of tumors in mice treated for 78 weeks at doses up to 200 mg/kg/day, which is approximately 16 times the trospium chloride dose at the MRHD, based on BSA.
Mutagenesis
Xanomeline
Xanomeline was not mutagenic in the in vitro bacterial reverse mutation (Ames assay) or mouse lymphoma assay. Xanomeline did not induce unscheduled DNA synthesis in rat hepatocytes and was not clastogenic in the in vitro chromosome aberration assay or in the in vivo mouse bone marrow micronucleus assay.Trospium chloride
Trospium chloride was not mutagenic nor genotoxic in tests in vitro in bacteria (Ames assay) and mammalian cells (L5178Y mouse lymphoma and CHO cells) or in vivo in the rat micronucleus test.Impairment of Fertility
Xanomeline
Xanomeline did not affect fertility when orally administered to male rats via the diet at doses of 15, 44, and 150 mg/kg/day. The NOAEL for male fertility is 150 mg/kg/day, which is approximately 6 times the xanomeline dose at the MRHD of 250/60 mg xanomeline/trospium, based on BSA.Xanomeline did not affect fertility when administered subcutaneously to male and female rats at doses of 1, 5, and 25 mg/kg/day. The NOAEL for male and female fertility is 25 mg/kg/day, which is equal to the xanomeline dose at the MRHD, based on BSA.
Trospium chloride
Trospium chloride did not affect fertility in rats at doses up to 200 mg/kg/day which is approximately 32 times the trospium chloride dose at the MRHD, based on BSA. -
14 CLINICAL STUDIES
The efficacy of COBENFY for the treatment of schizophrenia in adults was evaluated in two placebo-controlled studies with identical designs (N = 470). Study 1 (NCT04659161) and Study 2 (NCT04738123) were five-week, randomized, double-blind, placebo-controlled, multi-center studies in adult patients with a diagnosis of schizophrenia according to the DSM-5 criteria.
In Study 1 and Study 2, patients randomized to COBENFY were started on an initial dose of 50 mg/20 mg orally twice daily for the first 2 days and if tolerated, followed by 100 mg/20 mg orally twice daily for the remainder of Week 1 (Days 3 to 7). On Day 8, dosing was titrated upwards to 125 mg/30 mg orally twice daily unless the patient could not tolerate it. All patients could return to 100 mg/20 mg orally twice daily for the remainder of the treatment period.
Demographic and baseline disease characteristics were similar for the COBENFY and placebo groups. Median age was 46 years (range 19 to 65 years). Twenty-five percent of patients were female, 31% were White, 68% were Black or African American, and 1% were Other (or not reported).
The primary efficacy measure was the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score at Week 5. The PANSS is a 30-item scale that measures symptoms of schizophrenia. Each item is rated by a clinician on a seven-point scale. A score of 1 indicates the absence of symptoms, and a score of 7 indicates extremely severe symptoms. The PANSS total score may range from 30 to 210 with higher scores reflecting greater overall symptom severity.
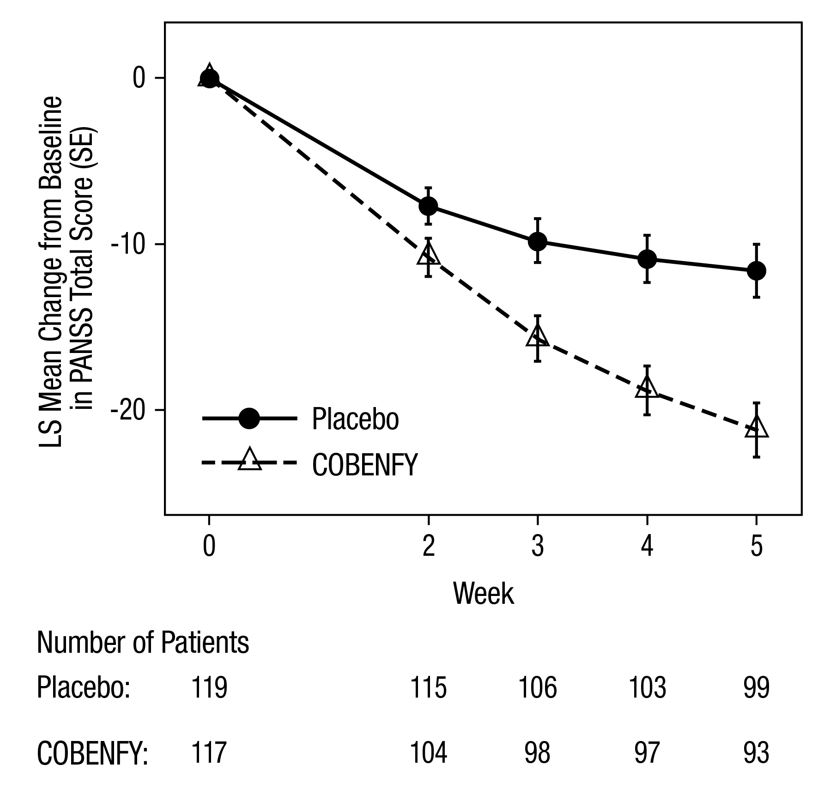
In Study 1 and Study 2, patients randomized to COBENFY showed a statistically significant reduction from baseline to Week 5 in the PANSS Total Score compared to the placebo group. The results of Studies 1 and 2 are shown in Table 4. A secondary endpoint, the change from baseline to Week 5 on the Clinical Global Impression‒Severity (CGI-S) score, was statistically significant for COBENFY compared to placebo in Study 1. The CGI-S is a validated clinician-rated scale that measures the patient’s current illness state and overall clinical state on a 1 (normal, not at all ill) to 7-point (extremely ill) scale.
Examination of subgroups by age, sex, and race did not suggest differences in response in the study (there were no patients over 65 years of age).
Table 4: Primary Efficacy Results for Change from Baseline in PANSS Total Score at Week 5 in Adults with Schizophrenia (Studies 1 and 2) Primary Efficacy Endpoint: PANSS Total Score
Study Number
Treatment Group
N
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference
(95% CI) a
1
COBENFY
117
98.2 (8.9)
-21.2 (1.7)
-9.6 (-13.9, -5.2)*
Placebo
119
97.7 (9.4)
-11.6 (1.6)
2
COBENFY
114
96.9 (8.8)
-20.6 (1.6)
-8.4 (-12.4, -4.3)*
Placebo
120
96.5 (8.8)
-12.2 (1.6)
The PANSS Total Score may range from 30 to 210; higher scores reflect greater symptom severity.
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
a Difference (drug minus placebo) in LS mean change from baseline.
*Statistically significantly superior to placebo.
The change from baseline in PANSS total score to Week 5 is summarized in Figure 1.
Figure 1: Change from Baseline in PANSS Total Score by Week in Adults with Schizophrenia (Study 1)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
COBENFY is available as:
- 50 mg/20 mg (xanomeline/trospium chloride): Buff capsules imprinted with Karuna 50/20 mg
- 100 mg/20 mg (xanomeline/trospium chloride): Brown capsules imprinted with Karuna 100/20 mg
- 125 mg/30 mg (xanomeline/trospium chloride): Swedish Orange capsules imprinted with Karuna 125/30 mg
COBENFY capsules are packaged as described in Table 5.
Table 5: COBENFY Packaging Configurations Capsule Strength
Total Package Count
Package Configuration
Package Components
NDC Code
50 mg/20 mg
60
Bottle
N/A
0003-0050-60
100
Hospital Unit-Dose Blister
10 Blister Cards with 10 Capsules Each
0003-0050-98
100 mg/20 mg
60
Bottle
N/A
0003-1100-60
100
Hospital Unit-Dose Blister
10 Blister Cards with 10 Capsules Each
0003-1100-98
125 mg/30 mg
60
Bottle
N/A
0003-0125-60
100
Hospital Unit-Dose Blister
10 Blister Cards with 10 Capsules Each
0003-0125-98
50 mg/20 mg (4)
100 mg/20 mg (52)
56
Starter Pack for 100 mg/20 mg dose
1 Mixed Blister Wallet: four (4) 50 mg/20 mg capsules and ten (10) 100 mg/20 mg capsules
and
3 Wallets: fourteen (14) 100 mg/20 mg capsules in each wallet
0003-5200-56
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Risk of Urinary Retention
Inform patients that COBENFY may cause urinary retention and that the risk of urinary retention is greater in some patients, including geriatric patients and those with bladder outlet obstruction (e.g., due to BPH) and patients with other causes of reduced bladder emptying (e.g., diabetic cystopathy). Urinary retention may occur at any time during treatment with COBENFY and is more likely when taking higher doses.Advise patients to monitor for symptoms of urinary retention, such as urinary hesitancy, weak urinary stream, incomplete bladder emptying and pain with urination, and to promptly report these symptoms to their healthcare provider. Inform patients that urinary retention may increase the risk of urinary tract infection. Advise patients to seek immediate medical attention if they are unable to urinate [see Warnings and Precautions (5.1)].
Risk of Use in Patients with Hepatic Impairment
Instruct patients to report signs of hepatic impairment (e.g., skin and eyes that appear yellowish, abdominal pain and swelling, itchy skin, dark urine color) and symptoms of hepatic injury (e.g., biliary spasm, pancreatitis, and cholangitis) to their healthcare provider [see Warnings and Precautions (5.2)].Risk of Use in Patients with Biliary Disease
Inform patients that COBENFY can increase liver enzymes and about the need for specific monitoring, including liver enzymes and bilirubin levels. Inform patients to report symptoms such as dyspepsia, nausea, vomiting, or upper abdominal pain to their healthcare provider [see Warnings and Precautions (5.3)].Decreased Gastrointestinal Motility
Inform patients that COBENFY can delay or slow emptying of food in their stomach. Advise patients to inform their healthcare provider about the presence of or symptoms of gastrointestinal obstructive disorders and conditions such as ulcerative colitis, intestinal atony, and myasthenia gravis [see Warnings and Precautions (5.4)].Risk of Angioedema
Advise patients that hypersensitivity reactions to COBENFY could and occur and result in life-threatening airway obstruction. Instruct patients to seek medical attention if they experience edema of the tongue, edema of the laryngopharynx, or difficulty breathing occurs, discontinue COBENFY, and seek immediate medical attention [see Warnings and Precautions (5.5)].Risk of Use in Patients with Narrow-angle Glaucoma
Inform patients pupillary dilation may occur with COBENFY use and in susceptible individuals, can lead to an episode of angle closure glaucoma [see Warnings and Precautions (5.6)].Increases in Heart Rate
Inform patients that COBENFY can increase heart rate [see Warnings and Precautions (5.7)].Anticholinergic Adverse Reactions in Patients with Renal Impairment
Inform patients that COBENFY is associated with anticholinergic adverse reactions such as dry mouth, constipation, dyspepsia, urinary tract infection, and urinary retention and the effects are expected to be greater in patients with renal impairment [see Warnings and Precautions (5.8)].Central Nervous System Effects
Advise patients that COBENFY is associated with central nervous system effects such as dizziness, confusion, hallucinations, and somnolence. Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that COBENFY therapy does not adversely affect their ability to engage in such activities [see Warnings and Precautions (5.9)].Administration Information
Instruct patients to take COBENFY twice daily at least one hour before a meal or at least 2 hours after a meal and not to open the capsules [see Dosage and Administration (2.2)].Concomitant Medications
Advise patients to inform their health care providers of any changes to their current prescription or over-the-counter medications because there may be a potential for interactions [see Drug Interactions (7)].Pregnancy
Advise patients to notify their healthcare provider with a known or suspected pregnancy. Advise pregnant women that there is a pregnancy exposure registry that monitors outcomes in females exposed to COBENFY during pregnancy [see Use in Specific Populations (8.1)].Marketed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
COBENFY is a trademark of Karuna Therapeutics, Inc., a Bristol Myers Squibb company. -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
COBENFY™ (co-BEN-fee)
(xanomeline and trospium chloride)
capsulesWhat is COBENFY?
COBENFY is a prescription medicine used to treat schizophrenia in adults.It is not known if COBENFY is safe and effective in children.
Do not take COBENFY if you:
- have urinary retention problems that cause your bladder to not empty completely or not empty at all
- have moderate or severe liver problems (impairment)
- have gastric retention problems that cause your stomach to empty slowly
- are allergic to COBENFY, xanomeline, or trospium chloride, or any of the ingredients in COBENFY. See the end of this Patient Information leaflet for a complete list of ingredients in COBENFY.
- have an eye problem called untreated narrow-angle glaucoma
Before taking COBENFY, tell your healthcare provider about all of your medical conditions, including if you:
- have an enlarged prostate, problems passing urine, or a blockage in your urinary bladder
- have liver problems
- have or had gallstones or problems with your bile ducts or pancreas
- have stomach or intestinal problems including constipation, ulcerative colitis, slow emptying of your stomach, or myasthenia gravis
- have an eye condition called narrow-angle glaucoma
- have kidney problems
-
are pregnant or plan to become pregnant. It is not known if COBENFY may harm your unborn baby. Tell your healthcare provider if you become pregnant or think you are pregnant during treatment with COBENFY.
- o There is a pregnancy exposure registry for women who take COBENFY during pregnancy. The purpose of this registry is to collect information about the health of women exposed to COBENFY and their baby. If you become pregnant during treatment with COBENFY, your healthcare provider will register you by calling 1-866-961-2388 or online at https://womensmentalhealth.org/research/pregnancyregistry/atypicalantipsychotic/.
- are breastfeeding or plan to breastfeed. It is not known if COBENFY passes into your breast milk or if it can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking COBENFY with certain other medicines may increase your risk of side effects from COBENFY or the other medicine and may affect the way COBENFY or the other medicine works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take COBENFY?
- Take COBENFY exactly as your healthcare provider tells you. Do not change the dose or stop taking COBENFY without first talking to your healthcare provider.
- Take 1 COBENFY capsule 2 times each day.
- Take COBENFY by mouth at least 1 hour before a meal or at least 2 hours after a meal.
- Do not open the capsules.
- If you take too much COBENFY, call your healthcare provider or Poison Help Line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while taking COBENFY?
Do not drive, operate heavy machinery, or do other dangerous activities until you know how COBENFY affects you. COBENFY may cause dizziness, confusion, seeing or hearing things that are not real (hallucinations), and sleepiness.
What are the possible side effects of COBENFY?
COBENFY may cause serious side effects, including:
- Problems with emptying your bladder (urinary retention). See “Do not take COBENFY if you:” COBENFY may cause your bladder to not empty completely or not empty at all. You are at increased risk for urinary retention if you are elderly, have a blockage in your bladder, have an enlarged prostate called benign prostatic hyperplasia (BPH), have bladder emptying problems from diabetes, or are taking higher doses of COBENFY. Urinary retention may increase your risk for getting a urinary tract infection. Call your healthcare provider or get emergency help right away if you get any signs or symptoms of urinary retention during treatment with COBENFY, including:
- o difficulty urinating
- o urination in a weak stream or drips
- o urinating frequently
- o full bladder and difficulty emptying your bladder
- o pain when you urinate
- Risks in people with liver problems. See “Do not take COBENFY if you:” It is not recommended that people with mild liver problems (impairment) take COBENFY because they have an increased risk of getting side effects from COBENFY. Your healthcare provider will check the liver enzyme levels in your blood before starting treatment and as needed during treatment with COBENFY. Tell your healthcare provider if you get any signs or symptoms of liver problems during treatment with COBENFY, including:
- o yellowing of your skin or the white part of your eyes
- o dark urine
- o pain and swelling in the upper right part of your stomach (abdomen)
- o stomach pain that spreads to your back or to below your right shoulder
- o itching
- o nausea or vomiting
- o loss of appetite
- o fever
- o chills
- o light colored stools
- o tiredness
- Risks in people with bile duct and gallbladder problems (biliary disease). COBENFY may cause a blockage in your bile ducts that could lead to gallstones, pancreatitis, and increases in your liver enzymes. Your healthcare provider will check your liver enzyme and bilirubin levels in your blood before starting treatment and as needed during treatment with COBENFY. Tell your healthcare provider if you get any signs or symptoms of biliary disorders during treatment with COBENFY, including:
- o stomach upset or burning (dyspepsia)
- o nausea
- o vomiting
- o pain in the upper right part of your stomach
- Slow emptying of your stomach (decreased gastrointestinal motility). See “Do not take COBENFY if you:” You are at increased risk for getting decreased gastrointestinal motility if you have ulcerative colitis, already have problems with slow stomach emptying, and have myasthenia gravis. Tell your healthcare provider if you get any signs and symptoms of decreased gastrointestinal motility during treatment with COBENFY, including:
- o constipation
- o vomiting
- o nausea
- o stomach (abdominal) bloating
- o stomach (abdominal) pain
- o a feeling of fullness after eating just a few bites
- o acid reflux
- Serious allergic reactions (angioedema). Angioedema may happen during treatment with COBENFY and can be life threatening. Stop taking COBENFY and call your healthcare provider or get emergency help right away if you get any of the following signs or symptoms of a serious allergic reaction during treatment with COBENFY, including:
- o hives
- o swelling of your face, lips, mouth, or tongue
- o swelling of your throat
- o hoarseness or difficulty speaking
- o breathing problems
- An eye problem called narrow-angle glaucoma. See “Do not take COBENFY if you:” If you already have narrow angles in your eyes, COBENFY may cause a sudden attack (acute angle closure) of glaucoma. Tell your healthcare provider if you get any signs or symptoms of narrow-angle glaucoma during treatment with COBENFY, including:
- o red eyes
- o blurred vision
- o seeing halos or bright colors around lights
- o eye pain or discomfort
- o nausea or vomiting
- o severe headache
- Increases in heart rate. COBENFY may increase your heart (pulse) rate. Your healthcare provider should check your heart rate before you start treatment and during treatment as needed. Tell your healthcare provider if you get a racing or pounding feeling in your chest during treatment with COBENFY.
- Side effects in people with kidney problems. People with kidney problems may have an increased risk of getting dry mouth, constipation, stomach upset or burning, urinary tract infection, and urinary retention during treatment with COBENFY.
- Central nervous system problems. See “What should I avoid while taking COBENFY?”
The most common side effects of COBENFY include:
- nausea
- stomach upset or burning (dyspepsia)
- constipation
- vomiting
- high blood pressure
- stomach (abdominal) pain
- diarrhea
- increased heart rate
- dizziness
- heartburn (gastrointestinal reflux disease)
Your healthcare provider may lower your dose or stop treatment with COBENFY if you get certain side effects.
These are not all of the possible side effects of COBENFY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store COBENFY?
- Store COBENFY at room temperature between 68°F to 77°F (20°C to 25°C).
Keep COBENFY and all medicines out of the reach of children.
General information about the safe and effective use of COBENFY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use COBENFY for a condition for which it was not prescribed. Do not give your COBENFY to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about COBENFY that is written for health professionals.
What are the ingredients in COBENFY?
Active ingredients: xanomeline and trospium chloride
Inactive ingredients: ascorbic acid, lactose monohydrate, microcrystalline cellulose, and talcThe capsule shell contains black iron oxide (only 100 mg/20 mg), hypromellose, red iron oxide, titanium dioxide, and yellow iron oxide (only 50 mg/20 mg and 100 mg/20 mg).
Marketed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
COBENFY is a trademark of Karuna Therapeutics, Inc., a Bristol Myers Squibb company.
For more information, go to www.COBENFY.com or call 1-800-721-5072.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 9/2024
-
PRINCIPAL DISPLAY PANEL - 50 mg/20 mg Capsule Bottle Label
NDC: 0003-0050-60
Rx OnlyCOBENFY™
(xanomeline and trospium chloride) capsulesRecommended
Dosage: Take one
capsule twice daily.
See Prescribing
Information50 mg/20 mg
60 Capsules -
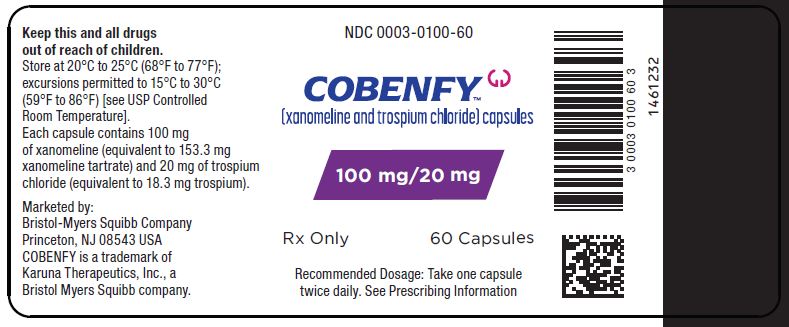
PRINCIPAL DISPLAY PANEL - 100 mg/20 mg Capsule Bottle Label
NDC: 0003-1100-60
COBENFY™
(xanomeline and trospium chloride) capsules100 mg/20 mg
Rx Only 60 CAPSULES
Recommended Dosage: Take one capsule
twice daily. See Prescribing Information -
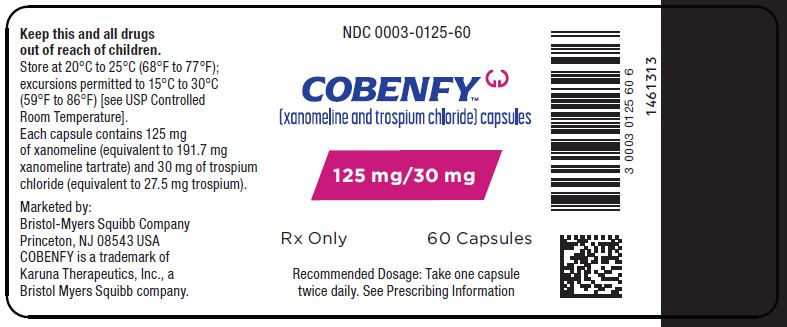
PRINCIPAL DISPLAY PANEL - 125 mg/30 mg Capsule Bottle Label
NDC: 0003-0125-60
Rx OnlyCOBENFY™
(xanomeline and trospium chloride) capsules125 mg/30 mg
Rx Only 60 Capsules
Recommended Dosage: Take on capsule
twice daily. See Prescribing Information -
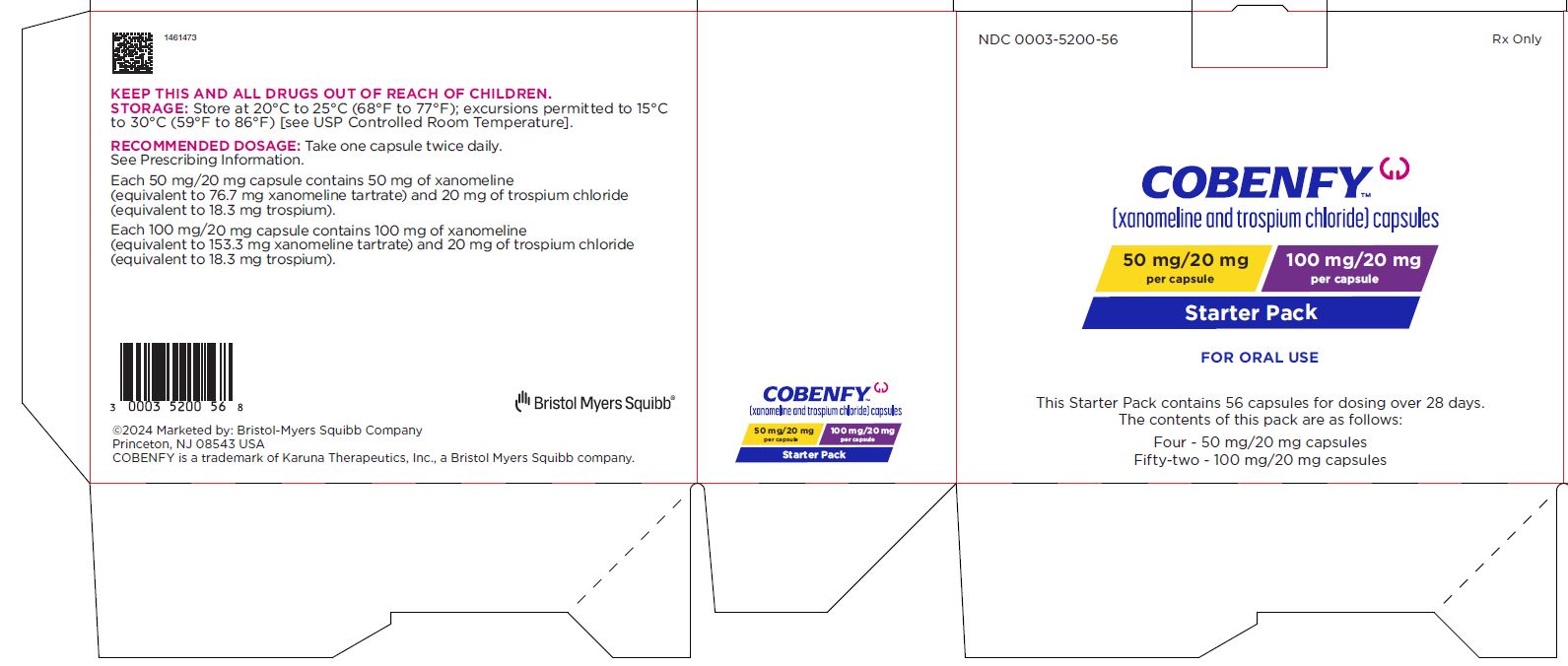
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 0003-5200-56
Rx Only
COBENFY™
(xanomeline and trospium chloride) capsules50 mg/20 mg
per capsule
100 mg/20 mg
per capsuleStarter Pack
FOR ORAL USE
This Starter Pack contains 56 capsules for dosing over 28 days.
The contents of this pack are as follows:
Four - 50 mg/20 mg capsules
Fifty-two - 100 mg/20 mg capsules -
PRINCIPAL DISPLAY PANEL - Sample Titration Wallet
PROFESSIONAL SAMPLE
NOT FOR RESALE
NDC: 0003-5150-14Rx Only
COBENFY™
(xanomeline and trospium chloride) capsules50 mg/20 mg
per capsule
100 mg/20 mg
per capsuleTITRATION PACK
Days 1-2: Take one capsule
(50 mg/20 mg) twice daily
Days 3-7: Take one capsule
(100 mg/20 mg) twice dailyContains 14 capsules:
Four - 50 mg/20 mg capsules
Ten - 100 mg/20 mg capsules -
INGREDIENTS AND APPEARANCE
COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-0050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 50 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (Light Tan) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Karuna;50;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-0050-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/27/2024 2 NDC: 0003-0050-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/27/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-1100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 100 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Karuna;100;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-1100-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/27/2024 2 NDC: 0003-1100-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/27/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-0125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 125 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 30 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) Product Characteristics Color ORANGE (Dark Orange) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Karuna;125;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-0125-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/27/2024 2 NDC: 0003-0125-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/27/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 COBENFY
xanomeline and trospium chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-5200 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-5200-56 1 in 1 CARTON; Type 0: Not a Combination Product 09/27/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 4 Part 2 1 BLISTER PACK 52 Part 1 of 2 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Item Code (Source) NDC: 0003-0050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 50 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (Light Tan) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Karuna;50;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-0050-04 1 in 1 PACKAGE 1 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 Part 2 of 2 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Item Code (Source) NDC: 0003-1100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 100 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Karuna;100;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-1100-01 1 in 1 PACKAGE 1 52 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 COBENFY
xanomeline and trospium chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-5150 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-5150-14 1 in 1 CARTON; Type 0: Not a Combination Product 09/27/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 4 Part 2 1 BLISTER PACK 10 Part 1 of 2 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Item Code (Source) NDC: 0003-0050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 50 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (Light Tan) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Karuna;50;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-0050-00 1 in 1 PACKAGE 1 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 Part 2 of 2 COBENFY
xanomeline and trospium chloride capsule, coated pelletsProduct Information Item Code (Source) NDC: 0003-1100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength xanomeline (UNII: 9ORI6L73CJ) (xanomeline - UNII:9ORI6L73CJ) xanomeline 100 mg trospium chloride (UNII: 1E6682427E) (trospium - UNII:T4Y8ORK057) trospium chloride 20 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) ascorbic acid (UNII: PQ6CK8PD0R) talc (UNII: 7SEV7J4R1U) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Karuna;100;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-1100-10 1 in 1 PACKAGE 1 10 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216158 09/27/2024 Labeler - E.R. Squibb & Sons, L.L.C. (011550092)
Trademark Results [Cobenfy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COBENFY 97333525 not registered Live/Pending |
Karuna Therapeutics, Inc. 2022-03-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.