tamoxifen citrate- Tamoxifen Citrate tablet
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING – For Women with Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer:Serious and life-threatening events associated with tamoxifen in the risk reduction setting (women at high risk for cancer and women with DCIS) include uterine malignancies, stroke and pulmonary embolism. Incidence rates for these events were estimated from the NSABP P-1 trial (see CLINICAL PHARMACOLOGY – Clinical Studies – Reduction in Breast Cancer Incidence in High Risk Women). Uterine malignancies consist of both endometrial adenocarcinoma (incidence rate per 1,000 women-years of 2.20 for tamoxifen vs. 0.71 for placebo) and uterine sarcoma (incidence rate per 1,000 women-years of 0.17 for tamoxifen vs. 0.04 for placebo)*. For stroke, the incidence rate per 1,000 women-years was 1.43 for tamoxifen vs. 1.00 for placebo**. For pulmonary embolism, the incidence rate per 1,000 women-years was 0.75 for tamoxifen versus 0.25 for placebo**.
Some of the strokes, pulmonary emboli, and uterine malignancies were fatal.
Health care providers should discuss the potential benefits versus the potential risks of these serious events with women at high risk of breast cancer and women with DCIS considering tamoxifen to reduce their risk of developing breast cancer.
The benefits of tamoxifen outweigh its risks in women already diagnosed with breast cancer.
*Updated long-term follow-up data (median length of follow-up is 6.9 years) from NSABP P-1 study. SeeWARNINGS: Effects on the Uterus - Endometrial Cancer and Uterine Sarcoma.
**See Table 3 underCLINICAL PHARMACOLOGY - Clinical Studies.
-
DESCRIPTION
Tamoxifen citrate tablets USP, a nonsteroidal antiestrogen, are for oral administration. Tamoxifen citrate tablets are available as:
10 mg Tablets. Each tablet contains 15.2 mg of tamoxifen citrate which is equivalent to 10 mg of tamoxifen.
20 mg Tablets. Each tablet contains 30.4 mg of tamoxifen citrate which is equivalent to 20 mg of tamoxifen.
Each tablet contains the following inactive ingredients: carboxymethylcellulose calcium, magnesium stearate, mannitol and starch.
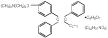
Chemically, tamoxifen is the trans-isomer of a triphenylethylene derivative. The chemical name is (Z)2-[4-(1,2-diphenyl-1-butenyl) phenoxy]-N, N-dimethylethanamine 2-hydroxy-1,2,3- propanetricarboxylate (1:1). The structural and molecular formulas are:
Tamoxifen citrate has a molecular weight of 563.62, the pKa' is 8.85, the equilibrium solubility in water at 37°C is 0.5 mg/mL and in 0.02 N HCl at 37°C, it is 0.2 mg/mL.
-
CLINICAL PHARMACOLOGY
Tamoxifen citrate is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Tamoxifen inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, tamoxifen appears to exert its antitumor effects by binding the estrogen receptors.
In cytosols derived from human breast adenocarcinomas, tamoxifen competes with estradiol for estrogen receptor protein.
Absorption and Distribution: Following a single oral dose of 20 mg tamoxifen, an average peak plasma concentration of 40 ng/mL (range 35 to 45 ng/mL) occurred approximately 5 hours after dosing. The decline in plasma concentrations of tamoxifen is biphasic with a terminal elimination half-life of about 5 to 7 days. The average peak plasma concentration of N-desmethyl tamoxifen is 15 ng/mL (range 10 to 20 ng/mL). Chronic administration of 10 mg tamoxifen given twice daily for 3 months to patients results in average steady-state plasma concentrations of 120 ng/mL (range 67-183 ng/mL) for tamoxifen and 336 ng/mL (range 148-654 ng/mL) for N-desmethyl tamoxifen. The average steady-state plasma concentrations of tamoxifen and N-desmethyl tamoxifen after administration of 20 mg tamoxifen once daily for 3 months are 122 ng/mL (range 71-183 ng/mL) and 353 ng/mL (range 152-706 ng/mL), respectively. After initiation of therapy, steady-state concentrations for tamoxifen are achieved in about 4 weeks and steady-state concentrations for N-desmethyl tamoxifen are achieved in about 8 weeks, suggesting a half-life of approximately 14 days for this metabolite. In a steady-state, crossover study of 10 mg tamoxifen citrate tablets given twice a day vs. a 20 mg tamoxifen citrate tablet given once daily, the 20 mg tamoxifen citrate tablet was bioequivalent to the 10 mg tamoxifen citrate tablets.
Metabolism: Tamoxifen is extensively metabolized after oral administration. N-desmethyl tamoxifen is the major metabolite found in patients' plasma. The biological activity of N-desmethyl tamoxifen appears to be similar to that of tamoxifen. 4-Hydroxytamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. Tamoxifen is a substrate of cytochrome P-450 3A, 2C9 and 2D6, and an inhibitor of P-glycoprotein.
Excretion: Studies in women receiving 20 mg of 14C tamoxifen have shown that approximately 65% of the administered dose was excreted from the body over a period of 2 weeks with fecal excretion as the primary route of elimination. The drug is excreted mainly as polar conjugates, with unchanged drug and unconjugated metabolites accounting for less than 30% of the total fecal radioactivity.
Special Populations: The effects of age, gender and race on the pharmacokinetics of tamoxifen have not been determined. The effects of reduced liver function on the metabolism and pharmacokinetics of tamoxifen have not been determined.
Pediatric Patients: Approved labeling describing pediatric pharmacokinetic information obtained from patients with McCune-Albright syndrome is available for AstraZeneca's tamoxifen citrate tablets. However, due to AstraZeneca's marketing exclusivity rights, this drug product is not labeled for pediatric use. The long-term effects of tamoxifen therapy in girls have not been established. In adults treated with tamoxifen citrate an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted (see BOXED WARNING).
Drug-drug Interactions:In vitro studies showed that erythromycin, cyclosporin, nifedipine and diltiazem competitively inhibited formation of N-desmethyl tamoxifen with apparent K1 of 20, 1, 45 and 30 μM, respectively. The clinical significance of these in vitro studies is unknown.
Tamoxifen reduced the plasma concentration of letrozole by 37% when these drugs were co-administered. Rifampin, a cytochrome P-450 3A4 inducer reduced tamoxifen AUC and Cmax by 86% and 55%, respectively. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
Clinical Studies--Metastatic Breast Cancer
Premenopausal Women (tamoxifen citrate vs. Ablation) --Three prospective, randomized studies (Ingle, Pritchard, Buchanan) compared tamoxifen to ovarian ablation (oophorectomy or ovarian irradiation) in premenopausal women with advanced breast cancer. Although the objective response rate, time to treatment failure, and survival were similar with both treatments, the limited patient accrual prevented a demonstration of equivalence. In an overview analysis of survival data from the 3 studies, the hazard ratio for death (tamoxifen /ovarian ablation) was 1.00 with two-sided 95% confidence intervals of 0.73 to 1.37. Elevated serum and plasma estrogens have been observed in premenopausal women receiving tamoxifen, but the data from the randomized studies do not suggest an adverse effect of this increase. A limited number of premenopausal patients with disease progression during tamoxifen therapy responded to subsequent ovarian ablation.
Male Breast Cancer --Published results from 122 patients (119 evaluable) and case reports in 16 patients (13 evaluable) treated with tamoxifen citrate have shown that tamoxifen is effective for the palliative treatment of male breast cancer. Sixty-six of these 132 evaluable patients responded to tamoxifen which constitutes a 50% objective response rate.
Clinical Studies--Adjuvant Breast Cancer
Overview --The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) conducted worldwide overviews of systemic adjuvant therapy for early breast cancer in 1985, 1990, and again in 1995. In 1998, 10-year outcome data were reported for 36,689 women in 55 randomized trials of adjuvant tamoxifen using doses of 20-40 mg/day for 1-5+ years. Twenty-five percent of patients received 1 year or less of trial treatment, 52% received 2 years, and 23% received about 5 years. Forty-eight percent of tumors were estrogen receptor (ER) positive (>10 fmol/mg), 21% were ER poor (<10 fmol/l), and 31% were ER unknown. Among 29,441 patients with ER positive or unknown breast cancer, 58% were entered into trials comparing tamoxifen to no adjuvant therapy and 42% were entered into trials comparing tamoxifen in combination with chemotherapy vs. the same chemotherapy alone. Among these patients, 54% had node positive disease and 46% had node negative disease.
Among women with ER positive or unknown breast cancer and positive nodes who received about 5 years of treatment, overall survival at 10 years was 61.4% for tamoxifen vs. 50.5% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 59.7% for tamoxifen vs. 44.5% for control (logrank 2p < 0.00001). Among women with ER positive or unknown breast cancer and negative nodes who received about 5 years of treatment, overall survival at 10 years was 78.9% for tamoxifen vs. 73.3% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 79.2% for tamoxifen versus 64.3% for control (logrank 2p < 0.00001).
The effect of the scheduled duration of tamoxifen may be described as follows. In women with ER positive or unknown breast cancer receiving 1 year or less, 2 years or about 5 years of tamoxifen, the proportional reductions in mortality were 12%, 17% and 26%, respectively (trend significant at 2p < 0.003). The corresponding reductions in breast cancer recurrence were 21%, 29% and 47% (trend significant at 2p < 0.00001).
Benefit is less clear for women with ER poor breast cancer in whom the proportional reduction in recurrence was 10% (2p = 0.007) for all durations taken together, or 9% (2p = 0.02) if contralateral breast cancers are excluded. The corresponding reduction in mortality was 6% (NS). The effects of about 5 years of tamoxifen on recurrence and mortality were similar regardless of age and concurrent chemotherapy. There was no indication that doses greater than 20 mg per day were more effective.
Node Positive--Individual Studies --Two studies (Hubay and NSABP B-09) demonstrated an improved disease-free survival following radical or modified radical mastectomy in postmenopausal women or women 50 years of age or older with surgically curable breast cancer with positive axillary nodes when tamoxifen was added to adjuvant cytotoxic chemotherapy. In the Hubay study, tamoxifen was added to "low-dose" CMF (cyclophosphamide, methotrexate and fluorouracil). In the NSABP B-09 study, tamoxifen was added to melphalan [L-phenylalanine mustard (P)] and fluorouracil (F).
In the Hubay study, patients with a positive (more than 3 fmol) estrogen receptor were more likely to benefit. In the NSABP B-09 study in women age 50-59 years, only women with both estrogen and progesterone receptor levels 10 fmol or greater clearly benefited, while there was a nonstatistically significant trend toward adverse effect in women with both estrogen and progesterone receptor levels less than 10 fmol. In women age 60-70 years, there was a trend toward a beneficial effect of tamoxifen without any clear relationship to estrogen or progesterone receptor status.
Three prospective studies (ECOG-1178, Toronto, NATO) using tamoxifen adjuvantly as a single agent demonstrated an improved disease-free survival following total mastectomy and axillary dissection for postmenopausal women with positive axillary nodes compared to placebo/no treatment controls. The NATO study also demonstrated an overall survival benefit.
Node Negative--Individual Studies --NSABP B-14, a prospective, double-blind, randomized study, compared tamoxifen to placebo in women with axillary node-negative, estrogen-receptor positive (≥ 10 fmol/mg cytosol protein) breast cancer (as adjuvant therapy, following total mastectomy and axillary dissection, or segmental resection, axillary dissection, and breast radiation). After five years of treatment, there was a significant improvement in disease-free survival in women receiving tamoxifen. This benefit was apparent both in women under age 50 and in women at or beyond age 50.
One additional randomized study (NATO) demonstrated improved disease-free survival for tamoxifen compared to no adjuvant therapy following total mastectomy and axillary dissection in postmenopausal women with axillary node-negative breast cancer. In this study, the benefits of tamoxifen appeared to be independent of estrogen receptor status.
Duration of Therapy --In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy.
In the NSABP B-14 trial, in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo and were disease-free at the end of this 5-year period were offered rerandomization to an additional 5 years of tamoxifen or placebo. With 4 years of follow-up after this rerandomization, 92% of the women that received 5 years of tamoxifen were alive and disease-free, compared to 86% of the women scheduled to receive 10 years of tamoxifen (p=0.003). Overall survivals were 96% and 94%, respectively (p=0.08). Results of the B-14 study suggest that continuation of therapy beyond 5 years does not provide additional benefit.
A Scottish trial of 5 years of tamoxifen vs. indefinite treatment found a disease-free survival of 70% in the five-year group and 61% in the indefinite group, with 6.2 years median follow-up (HR=1.27, 95% CI 0.87-1.85).
In a large randomized trial conducted by the Swedish Breast Cancer Cooperative Group of adjuvant tamoxifen 40 mg/day for 2 or 5 years, overall survival at 10 years was estimated to be 80% in the patients in the 5-year tamoxifen group, compared with 74% among corresponding patients in the 2-year treatment group (p=0.03). Disease-free survival at 10 years was 73% in the 5-year group and 67% in the 2-year group (p=0.009). Compared with 2 years of tamoxifen treatment, 5 years of treatment resulted in a slightly greater reduction in the incidence of contralateral breast cancer at ten years, but this difference was not statistically significant.
Contralateral Breast Cancer --The incidence of contralateral breast cancer is reduced in breast cancer patients (premenopausal and post-menopausal) receiving tamoxifen compared to placebo. Data on contralateral breast cancer are available from 32,422 out of 36,689 patients in the 1995 overview analysis of the Early Breast Cancer Trialists Collaborative Group (EBCTCG). In clinical trials with tamoxifen of 1 year or less, 2 years, and about 5 years duration, the proportional reductions in the incidence rate of contralateral breast cancer among women receiving tamoxifen were 13% (NS), 26% (2p = 0.004) and 47% (2p < 0.00001), with a significant trend favoring longer tamoxifen duration (2p = 0.008). The proportional reductions in the incidence of contralateral breast cancer were independent of age and ER status of the primary tumor. Treatment with about 5 years of tamoxifen reduced the annual incidence rate of contralateral breast cancer from 7.6 per 1,000 patients in the control group compared with 3.9 per 1,000 patients in the tamoxifen group.
In a large randomized trial in Sweden (the Stockholm Trial) of adjuvant tamoxifen 40 mg/day for 2-5 years, the incidence of second primary breast tumors was reduced 40% (p < 0.008) on tamoxifen compared to control. In the NSABP B-14 trial in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo, the incidence of second primary breast cancers was also significantly reduced (p < 0.01). In NSABP B-14, the annual rate of contralateral breast cancer was 8.0 per 1,000 patients in the placebo group compared with 5.0 per 1,000 patients in the tamoxifen group, at 10 years after first randomization.
Clinical Studies – Ductal Carcinoma in Situ: NSABP B-24, a double-blind, randomized trial included women with ductal carcinoma in situ (DCIS). This trial compared the addition of tamoxifen or placebo to treatment with lumpectomy and radiation therapy for women with DCIS. The primary objective was to determine whether 5 years of tamoxifen therapy (20 mg/day) would reduce the incidence of invasive breast cancer in the ipsilateral (the same) or contralateral (the opposite) breast.
In this trial 1,804 women were randomized to receive either tamoxifen or placebo for 5 years: 902 women were randomized to tamoxifen 10 mg tablets twice a day and 902 women were randomized to placebo. As of December 31, 1998, the follow-up data were available 1,798 women and the median duration of the follow-up was 74 months.
The tamoxifen and placebo groups were well balanced for baseline demographic and prognostic factors. Over 80% of the tumors were less than or equal to 1 cm in their maximum dimension, were not palpable, and were detected by mammography alone. Over 60% of the study population was postmenopausal. In 16% of patients, the margin of the resected specimen was reported as being positive after surgery. Approximately half of the tumors were reported to contain comedo necrosis.
For the primary endpoint, the incidence of invasive breast cancer was reduced 43% among women assigned to tamoxifen (44 cases – tamoxifen, 74 cases – placebo; p=0.004; relative risk (RR)=0.57, 95% CI: 0.39-0.84). No data are available regarding the ER status of the invasive cancers. The stage distribution of the invasive cancers at diagnosis was similar to that reported annually in the SEER data base.
Results are shown in Table 1. For each endpoint the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.
Table 1 - Major Outcomes of the NSABP B-24 Trial 1Updated follow-up data (median 8.1 years)
Type of Event Lumpectomy,
radiotherapy, and placeboLumpectomy,
radiotherapy, and
tamoxifenRR 95% CI Limits No. of Events Rate per
1000 women
per yearNo. of Events Rate per
1000 women
per yearInvasion breast cancer
(Primary endpoint)74 16.73 44 9.60 0.57 0.39 to 0.84 lpsilateral 47 10.61 27 5.90 0.56 0.33 to 0.91 Contralateral 25 5.64 17 3.71 0.66 0.33 to 1.27 Side undetermined 2 — 0 — — Secondary Endpoint DCIS 56 12.66 41 8.95 0.71 0.46 to 1.08 lpsilateral 46 10.40 38 8.29 0.88 0.51 to 1.25 Contralateral 10 2.26 3 0.65 0.29 0.05 to 1.13 All Breast Cancer Events 129 29.16 84 18.34 0.63 0.47 to 0.83 All ipsilateral events 96 21.70 65 14.19 0.65 0.47 to 0.91 All contralateral events 37 8.36 20 4.37 0.52 0.29 to 0.92 Deaths 32 28 Uterine Malignancies1 4 9 Endometrial Adenocarcinoma1 4 0.57 8 1.15 Uterine Sarcoma1 0 0.0 1 0.14 Second primaly malignancies
(other than endometrial and breast)30 29 Stroke 2 7 Thromboembolic events
(DVT, PE)5 15 Survival was similar in the placebo and the tamoxifen groups. At 5 years from study entry, survival was 97% for both groups.
Clinical Studies--Reduction in Breast Cancer Incidence in High Risk Women
The Breast Cancer Prevention Trial (BCPT, NSABP P-1) was a double-blind, randomized, placebo-controlled trial with a primary objective to determine whether five years of tamoxifen citrate therapy (20 mg/day) would reduce the incidence of invasive breast cancer in women at high risk for the disease (See INDICATIONS AND USAGE). Secondary objectives included an evaluation of the incidence of ischemic heart disease; the effects on the incidence of bone fractures; and other events that might be associated with the use of tamoxifen, including: endometrial cancer, pulmonary embolus, deep vein thrombosis, stroke, and cataract formation and surgery (See WARNINGS).
The Gail Model was used to calculate predicted breast cancer risk for women who were less than 60 years of age and did not have lobular carcinoma in situ (LCIS). The following risk factors were used: age; number of first-degree female relatives with breast cancer; previous breast biopsies; presence or absence of atypical hyperplasia; nulliparity; age at first live birth; and age at menarche. A 5-year predicted risk of breast cancer of ≥1.67% was required for entry into the trial.
In this trial, 13,388 women of at least 35 years of age were randomized to receive either tamoxifen or placebo for 5 years. The median duration of treatment was 3.5 years. As of January 31, 1998, follow-up data is available for 13,114 women. Twenty-seven percent of women randomized to placebo (1,782) and 24% of women randomized to tamoxifen (1,596) completed 5 years of therapy. The demographic characteristics of women on the trial with follow-up data are shown in Table 2.
Table 2 – Demographic Characteristics of Women in the NSABP P-1 Trial Placebo Tamoxifen Characteristic Placebo Tamoxifen Characteristic # % # % # % # % Age (yrs.) Prior Hysterectomy 35-39 184 3 158 2 No 4,173 63.5 4,018 62.4 40-49 2,394 36 2,411 37 Yes 2,397 36.5 2,464 37.7 50-59 2,011 31 2,019 31 # of previous breast biopsies 60-69 1,588 24 1,563 24 0 2,935 45 2,923 45 ≥70 393 6 393 6 1 1,8733 28 1,850 28 Age at first live birth (yrs.) ≥2 1,802 27 1,771 27 Nulliparous 1,202 18 1,205 18 History of atypical hyperplasia in the breast 12-19 915 14 946 15 No 5,958 91 5,969 91 20-24 2,448 37 2,449 37 Yes 612 9 575 9 26-29 1,399 21 1,367 21 ≥30 606 9 577 9 History of LCIS at entry Race No 6,165 94 6,135 94 White 6,333 96 6,323 96 Yes 405 6 409 6 Black 109 2 103 2 5-year predicted breast cancer risk (%) Other 128 2 118 2 ≤2.00 1,646 25 1,626 25 Age at menarche 2.01-3.00 2,028 31 2,057 31 ≥14 1,243 19 1,170 18 3.01-5.00 1,787 27 1,707 26 12-13 3,610 55 3,610 55 ≥5.01 1,109 17 1,162 18 ≤11 1,717 26 1,764 27 # of first degree relatives with breast cancer TOTAL 6,570 100.0 6,544 100.0 0 1,584 24 1,525 23 1 3,714 57 3,744 57 2+ 1,272 19 1,275 20 Results are shown in Table 3. After a median follow-up of 4.2 years, the incidence of invasive breast cancer was reduced by 44% among women assigned to tamoxifen (86 cases- tamoxifen, 156 cases-placebo; p<0.00001; relative risk (RR)=0.56, 95% CI: 0.43-0.72). A reduction in the incidence of breast cancer was seen in each prospectively specified age group (≤49, 50-59, ≥60), in women with or without LCIS, and in each of the absolute risk levels specified in Table 3. A non-significant decrease in the incidence of ductal carcinoma in situ (DCIS) was seen (23- tamoxifen, 35-placebo; RR=0.66; 95% CI: 0.39-1.11).
There was no statistically significant difference in the number of myocardial infarctions, severe angina, or acute ischemic cardiac events between the two groups (61- tamoxifen citrate, 59-placebo; RR=1.04, 95% CI: 0.73-1.49).
No overall difference in mortality (53 deaths in tamoxifen group vs. 65 deaths in placebo group) was present. No difference in breast cancer-related mortality was observed (4 deaths in tamoxifen group vs. 5 deaths in placebo group).
Although there was a non-significant reduction in the number of hip fractures (9 on tamoxifen, 20 on placebo) in the tamoxifen group, the number of wrist fractures was similar in the two treatment groups (69 on tamoxifen, 74 on placebo). No information regarding bone mineral density or other markers of osteoporosis is available.
The risks of tamoxifen therapy include endometrial cancer, DVT, PE, stroke, cataract formation and cataract surgery (See Table 3). In the NSABP P-1 trial, 33 cases of endometrial cancer were observed in the tamoxifen group vs. 14 in the placebo group (RR=2.48, 95% CI: 1.27-4.92). Deep vein thrombosis was observed in 30 women receiving tamoxifen vs. 19 in women receiving placebo (RR=1.59, 95% CI: 0.86-2.98). Eighteen cases of pulmonary embolism were observed in the tamoxifen group vs. 6 in the placebo group (RR=3.01, 95% CI: 1.15-9.27). There were 34 strokes on the tamoxifen arm and 24 on the placebo arm (RR=1.42; 95% CI: 0.82-2.51). Cataract formation in women without cataracts at baseline was observed in 540 women taking tamoxifen vs. 483 women receiving placebo (RR=1.13, 95% CI: 1.00-1.28). Cataract surgery (with or without cataracts at baseline) was performed in 201 women taking tamoxifen vs. 129 women receiving placebo (RR=1.51, 95% CI 1.21-1.89) (See WARNINGS).
Table 3 summarizes the major outcomes of the NSABP P-1 trial. For each endpoint, the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits or risks of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.
For most participants, multiple risk factors would have been required for eligibility. This table considers risk factors individually, regardless of other co-existing risk factors, for women who developed breast cancer. The 5-year predicted absolute breast cancer risk accounts for multiple risk factors in an individual and should provide the best estimate of individual benefit (See INDICATIONS AND USAGE).
Table 3 – Major Outcomes of the NSABP P-1 Trial 1 Two women had hip and wrist fractures
2 Includes Colles' and other lower radius fractures
3 Requiring angioplasty or CABG
4 New Q-wave on ECG; no angina or elevation of serum enzymes; or angina requiring hospitalization without surgery
5 Seven cases were fatal; three in the placebo group and four in the tamoxifen group
6 Three cases in the tamoxifen group were fatal
7 All but three cases in each group required hospitalization
8 Based on women without cataracts at baseline (6,230-Placebo, 6,199- tamoxifen)
9 All women (6,707-Placebo, 6,681-tamoxifen)
10 Updated long-term follow-up data (median 6.9 years) from NSABP P-1 study added after cut-off for the other information in this table.
# Of Events Rate/1000 women/Year 95% CI Type of Event Placebo Tamoxifen Placebo Tamoxifen RR Limits Invasive Breast Cancer 156 86 6.49 3.58 0.56 0.43-0.72 Age ≤49 59 38 6.34 4.11 0.65 0.43-0.98 Age 50-59 46 25 6.31 3.53 0.56 0.35-0.91 Age ≥60 51 23 7.17 3.22 0.45 0.27-0.74 Risk Factors for Breast Cancer History. LCIS No 140 78 6.23 3.51 0.56 0.43-0.74 Yes 16 8 12.73 6.33 0.50 0.21-1.17 History. Atypical Hyperplasia No 138 84 6.37 3.89 0.61 0.47-0.80 Yes 18 2 8.69 1.05 0.12 0.03-0.52 # First Degree Relatives 0 32 17 5.97 3.26 0.55 0.30-0.98 1 80 45 5.81 3.31 0.57 0.40-0.82 2 35 18 8.92 4.67 0.52 0.30-0.92 ≥3 9 6 13.33 7.58 0.57 0.20-1.59 5-Year Predicted Breast Cancer Risk (as calculated by the Gail Model) ≤2.00% 31 13 5.36 2.26 0.42 0.22-0.81 2.01-3.00% 39 28 5.25 3.83 0.73 0.45-1.18 3.01-5.00% 36 26 5.37 4.06 0.76 0.46-1.26 ≥5.00% 50 19 13.15 4.71 0.36 0.21-0.61 DCIS 35 23 1.47 0.97 0.66 0.39-1.11 Fractures (protocol-specified sites) 92 1 76 1 3.87 3.20 0.61 0.83-1.12 Hip 20 9 0.84 0.38 0.45 0.18-1.04 Wrist2 74 69 3.11 2.91 0.93 0.67-1.29 Total Ischemic Events 59 61 2.47 2.57 1.04 0.71-1.51 Myocardial Infarction 27 27 1.13 1.13 1.00 0.57-1.78 Fatal 8 7 0.33 0.29 0.88 0.27-2.77 Nonfatal 19 20 0.79 0.84 1.06 0.54-2.09 Angina 3 12 12 0.50 0.50 1.00 0.41-2.44 Acute Ischemic Syndrome 4 20 22 0.84 0.92 1.11 0.58-2.13 Uterine Malignancies (among women 17 57 with an intact uterus)10 Endometrial Adenocarcinoma10 17 53 0.71 2.20 Uterine Sarcoma10 0 4 0.0 0.17 Stroke5 24 34 1.00 1.43 1.42 0.82-2.51 Transient Ischemic Attack 21 18 0.88 0.75 0.86 0.43-1.70 Pulmonary Emboli6 6 18 0.25 0.75 3.01 1.15-9.27 Deep-Vein Thrombosis7 19 30 0.79 1.26 1.59 0.86-2.98 Cataracts Developing on Study8 483 540 22.51 25.41 1.13 1.00-1.28 Underwent Cataract Surgery8 63 101 21.83 4.57 1.62 1.18-2.22 Underwent Cataract Surgery9 129 201 5.44 8.56 1.58 1.26-1.97 Table 4 describes the characteristics of the breast cancers in the NSABP P-1 trial and includes tumor size, nodal status, ER status. Tamoxifen decreased the incidence of small estrogen receptor positive tumors, but did not alter the incidence of estrogen receptor negative tumors or larger tumors.
Table 4 – Characteristics of Breast Cancer in NSABP P-1 Trial 1 One participant presented with a suspicious bone scan but did not have documented metastases. She subsequently died of metastatic breast cancer.
Placebo Tamoxifen Total Placebo Tamoxifen Total Staging Parameter N=156 N=86 N=242 Staging Parameter N=156 N=86 N=242 Tumor Size: Stage: T1 117 60 177 I 88 47 135 T2 28 20 48 II: node negative 15 9 24 T3 7 3 10 II: node positive 33 22 55 T4 1 2 3 III 6 4 10 Unknown 3 1 4 IV 21 1 3 Unknown 12 3 15 Nodal Status: Negative 103 56 159 Estrogen receptor: 1-3 positive nodes 29 14 43 Positive 115 38 153 ≥ 4 positive nodes 10 12 22 Negative 27 36 63 Unknown 14 4 18 Unknown 14 12 26 Interim results from 2 trials in addition to the NSABP P-1 trial examining the effects of tamoxifen in reducing breast cancer incidence have been reported.
The first was the Italian Tamoxifen Prevention trial. In this trial women between the ages of 35 and 70, who had had a total hysterectomy, were randomized to receive 20 mg tamoxifen or matching placebo for 5 years. The primary endpoints were occurrence of, and death from, invasive breast cancer. Women without any specific risk factors for breast cancer were to be entered. Between 1992 and 1997, 5,408 women were randomized. Hormone Replacement Therapy (HRT) was used in 14% of participants. The trial closed in 1997 due to the large number of dropouts during the first year of treatment (26%). After 46 months of follow-up there were 22 breast cancers in women on placebo and 19 in women on tamoxifen. Although no decrease in breast cancer incidence was observed, there was a trend for a reduction in breast cancer among women receiving protocol therapy for at least 1 year (19-placebo, 11-tamoxifen). The small numbers of participants along with the low level of risk in this otherwise healthy group precluded an adequate assessment of the effect of tamoxifen in reducing the incidence of breast cancer.
The second trial, the Royal Marsden Trial (RMT) was reported as an interim analysis. The RMT was begun in 1986 as a feasibility study of whether larger scale trials could be mounted. The trial was subsequently extended to a pilot trial to accrue additional participants to further assess the safety of tamoxifen. Twenty-four hundred and seventy-one women were entered between 1986 and 1996; they were selected on the basis of a family history of breast cancer. HRT was used in 40% of participants. In this trial, with a 70-month median follow-up, 34 and 36 breast cancers (8 noninvasive, 4 on each arm) were observed among women on tamoxifen and placebo, respectively. Patients in this trial were younger than those in the NSABP P-1 trial and may have been more likely to develop ER (-) tumors, which are unlikely to be reduced in number by tamoxifen therapy. Although women were selected on the basis of family history and were thought to have a high risk of breast cancer, few events occurred, reducing the statistical power of the study. These factors are potential reasons why the RMT may not have provided an adequate assessment of the effectiveness of tamoxifen in reducing the incidence of breast cancer.
In these trials, an increased number of cases of deep vein thrombosis, pulmonary embolus, stroke, and endometrial cancer were observed on the tamoxifen arm compared to the placebo arm. The frequency of events was consistent with the safety data observed in the NSABP P-1 trial.
Clinical Studies-McCune-Albright: Approved labeling describing a pediatric clinical study regarding tamoxifen use in patients with McCune-Albright syndrome is available for AstraZeneca's tamoxifen citrate tablets. However, due to AstraZeneca's marketing exclusivity rights, this drug product is not labeled for pediatric use. The long-term effects of tamoxifen therapy in girls have not been established. Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted in adults treated with tamoxifen (see BOXED WARNING), continued monitoring of McCune-Albright patients treated with tamoxifen for long-term uterine effects is recommended.
-
INDICATIONS AND USAGE
Metastatic Breast Cancer: Tamoxifen citrate tablets are effective in the treatment of metastatic breast cancer in women and men. In premenopausal women with metastatic breast cancer, tamoxifen is an alternative to oophorectomy or ovarian irradiation. Available evidence indicates that patients whose tumors are estrogen receptor positive are more likely to benefit from tamoxifen therapy.
Adjuvant Treatment of Breast Cancer: Tamoxifen citrate tablets are indicated for the treatment of node-positive breast cancer in postmenopausal women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation. In some tamoxifen adjuvant studies, most of the benefit to date has been in the subgroup with 4 or more positive axillary nodes.
Tamoxifen is indicated for the treatment of axillary node-negative breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation.
The estrogen and progesterone receptor values may help to predict whether adjuvant tamoxifen therapy is likely to be beneficial.
Tamoxifen reduces occurrence of contralateral breast cancer in patients receiving adjuvant tamoxifen therapy for breast cancer.
Ductal Carcinoma in Situ (DCIS): In women with DCIS, following breast surgery and radiation, tamoxifen citrate tablets are indicated to reduce the risk of invasive breast cancer (seeBOXED WARNING at the beginning of the label). The decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy.
Current data from clinical trials support five years of adjuvant tamoxifen therapy for patients with breast cancer.
Reduction in Breast Cancer Incidence in High Risk Women: Tamoxifen citrate tablets are indicated to reduce the incidence of breast cancer in women at high risk for breast cancer. This effect was shown in a study of 5 years planned duration with a median follow-up of 4.2 years. Twenty-five percent of the participants received drug for 5 years. The longer-term effects are not known. In this study, there was no impact of tamoxifen on overall or breast cancer-related mortality (seeBOXED WARNING at the beginning of the label).
Tamoxifen citrate tablets are indicated only for high-risk women. "High risk" is defined as women at least 35 years of age with a 5-year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
Examples of combinations of factors predicting a 5-year risk ≥ 1.67% are:
Age 35 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer, 2 or more benign biopsies, and a history of a breast biopsy showing atypical hyperplasia; or
- At least 2 first degree relatives with a history of breast cancer, and a personal history of at least 1 breast biopsy; or
- LCIS
Age 40 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer, 2 or more benign biopsies, age at first live birth 25 or older, and age at menarche 11 or younger; or
- At least 2 first degree relatives with a history of breast cancer, and age at first live birth 19 or younger; or
- One first degree relative with a history of breast cancer, and a personal history of a breast biopsy showing atypical hyperplasia.
Age 45 or older and any of the following combination of factors:
- At least 2 first degree relatives with history of breast cancer and age at first live birth 24 or younger; or
- One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, age at menarche 11 or less and age at first live birth 20 or more.
Age 50 or older and any of the following combination of factors:
- At least 2 first degree relatives with a history of breast cancer; or
- History of one breast biopsy showing atypical hyperplasia, and age at first live birth 30 or older and age at menarche 11 or less; or
- History of at least two breast biopsies with a history of atypical hyperplasia, and age at first live birth 30 or more.
Age 55 or older and any of the following combination of factors:
- One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, and age at menarche 11 or less; or
- History of at least 2 breast biopsies with a history of atypical hyperplasia, and age at first live birth 20 or older.
Age 60 or older and:
- 5-year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
For women whose risk factors are not described in the above examples, the Gail Model is necessary to estimate absolute breast cancer risk. Health Care Professionals can obtain a Gail Model Risk Assessment Tool by dialing 1-866-292-6719.
There are no data available regarding the effect of tamoxifen on breast cancer incidence in women with inherited mutations (BRCA1, BRCA2).
After an assessment of the risk of developing breast cancer, the decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy. In the NSABP P-1 trial, tamoxifen treatment lowered the risk of developing breast cancer during the follow-up period of the trial, but did not eliminate breast cancer risk (See Table 3 in CLINICAL PHARMACOLOGY).
-
CONTRAINDICATIONS
Tamoxifen citrate tablets are contraindicated in patients with known hypersensitivity to the drug or any of its ingredients.
Reduction in Breast Cancer Incidence in High Risk Women and Women with DCIS: Tamoxifen citrate tablets are contraindicated in women who require concomitant coumarin-type anticoagulant therapy or in women with a history of deep vein thrombosis or pulmonary embolus.
-
WARNINGS
Effects in Metastatic Breast Cancer Patients: As with other additive hormonal therapy (estrogens and androgens), hypercalcemia has been reported in some breast cancer patients with bone metastases within a few weeks of starting treatment with tamoxifen. If hypercalcemia does occur, appropriate measures should be taken and, if severe, tamoxifen should be discontinued.
Effects on the Uterus-Endometrial Cancer and Uterine Sarcoma: An increased incidence of uterine malignancies has been reported in association with tamoxifen treatment. The underlying mechanism is unknown, but may be related to the estrogen-like effect of tamoxifen. Most uterine malignancies seen in association with tamoxifen are classified as adenocarcinoma of the endometrium. However, rare uterine sarcomas, including malignant mixed mullerian tumors, have also been reported. Uterine sarcoma is generally associated with a higher FIGO stage (III/IV) at diagnosis, poorer prognosis, and shorter survival. Uterine sarcoma has been reported to occur more frequently among long-term users (≥ 2 years) of tamoxifen than non-users. Some of the uterine malignancies (endometrial carcinoma or uterine sarcoma) have been fatal.
In the NSABP P-1 trial, among participants randomized to tamoxifen there was a statistically significant increase in the incidence of endometrial cancer (33 cases of invasive endometrial cancer, compared to 14 cases among participants randomized to placebo (RR=2.48, 95% CI: 1.27-4.92). The 33 cases in participants receiving tamoxifen were FIGO Stage I, including 20 IA, 12 IB and 1 IC endometrial adenocarcinomas. In participants randomized to placebo, 13 were FIGO Stage I (8 IA and 5 IB) and 1 was FIGO Stage IV. Five women on tamoxifen and 1 on placebo received postoperative radiation therapy in addition to surgery. This increase was primarily observed among women at least 50 years of age at the time of randomization (26 cases of invasive endometrial cancer, compared to 6 cases among participants randomized to placebo (RR=4.50, 95% CI: 1.78-13.16). Among women ≤ 49 years of age at the time of randomization there were 7 cases of invasive endometrial cancer, compared to 8 cases among participants randomized to placebo (RR=0.94, 95% CI: 0.28-2.89). If age at the time of diagnosis is considered, there were 4 cases of endometrial cancer among participants ≤ 49 randomized to tamoxifen compared to 2 among participants randomized to placebo (RR=2.21, 95% CI: 0.4-12.0). For women ≥ 50 at the time of diagnosis, there were 29 cases among participants randomized to tamoxifen compared to 12 among women on placebo (RR=2.5, 95% CI: 1.3-4.9). The risk ratios were similar in the two groups, although fewer events occurred in younger women. Most (29 of 33 cases in the tamoxifen group) endometrial cancers were diagnosed in symptomatic women, although 5 of 33 cases in the tamoxifen group occurred in asymptomatic women. Among women receiving tamoxifen the events appeared between 1 and 61 months (average = 32 months) from the start of treatment.
In an updated review of long-term data (median length of total follow-up is 6.9 years, including blinded follow-up) on 8,306 women with an intact uterus at randomization in the NSABP P-1 risk reduction trial, the incidence of both adenocarcinomas and rare uterine sarcomas was increased in women taking tamoxifen. During blinded follow-up, there were 36 cases of FIGO Stage I endometrial adenocarcinoma (22 were FIGO IA, 13 IB, and 1 IC) in women receiving tamoxifen and 15 cases in women receiving placebo [14 were FIGO Stage I (9 IA and 5 IB), and 1 case was FIGO Stage IV]. Of the patients receiving tamoxifen who developed endometrial cancer, one with Stage IA and 4 with Stage IB cancers received radiation therapy. In the placebo group, one patient with FIGO Stage IB cancer received radiation therapy and the patient with FIGO Stage IVB cancer received chemotherapy and hormonal therapy. During total follow-up, endometrial adenocarcinoma was reported in 53 women randomized to tamoxifen (30 cases of FIGO Stage IA, 20 were Stage IB, 1 was Stage IC, and 2 were Stage IIIC) and 17 women randomized to placebo (9 cases were FIGO Stage IA, 6 were Stage IB, 1 was Stage IIIC, and 1 was Stage IVB (incidence per 1,000 women-years of 2.20 and 0.71, respectively). Some patients received post-operative radiation therapy in addition to surgery. Uterine sarcomas were reported in 4 women randomized to tamoxifen (1 FIGO IA, 1 FIGO IB, 1 FIGO IIA, and 1 FIGO IIIC) and one patient randomized to placebo (FIGO 1A); incidence per 1,000 women-years of 0.17 and 0.04, respectively. Of the patients randomized to tamoxifen, the FIGO IA and IB cases were a MMMT and sarcoma, respectively; the FIGO II was a MMMT; and the FIGO III was a sarcoma) and the one patient randomized to placebo had a MMMT. A similar incidence in endometrial adenocarcinoma and uterine sarcoma was observed among women receiving tamoxifen in five other NSABP clinical trials.
Any patient receiving or who has previously received tamoxifen who reports abnormal vaginal bleeding should be promptly evaluated. Patients receiving or who have previously received tamoxifen should have annual gynecological examinations and they should promptly inform their physicians if they experience any abnormal gynecological symptoms, eg, menstrual irregularities, abnormal vaginal bleeding, changes in vaginal discharge, or pelvic pain or pressure.
In the P-1 trial, endometrial sampling did not alter the endometrial cancer detection rate compared to women who did not undergo endometrial sampling (0.6% with sampling, 0.5% without sampling) for women with an intact uterus. There are no data to suggest that routine endometrial sampling in asymptomatic women taking tamoxifen to reduce the incidence of breast cancer would be beneficial.
Non-Malignant Effects on the Uterus: An increased incidence of endometrial changes including hyperplasia and polyps have been reported in association with tamoxifen treatment. The incidence and pattern of this increase suggest that the underlying mechanism is related to the estrogenic properties of tamoxifen.
There have been a few reports of endometriosis and uterine fibroids in women receiving tamoxifen. The underlying mechanism may be due to the partial estrogenic effect of tamoxifen. Ovarian cysts have also been observed in a small number of premenopausal patients with advanced breast cancer who have been treated with tamoxifen.
Tamoxifen has been reported to cause menstrual irregularity or amenorrhea.
Thromboembolic Effects of Tamoxifen: There is evidence of an increased incidence of thromboembolic events, including deep vein thrombosis and pulmonary embolism, during tamoxifen therapy. When tamoxifen is coadministered with chemotherapy, there may be a further increase in the incidence of thromboembolic effects. For treatment of breast cancer, the risks and benefits of tamoxifen should be carefully considered in women with a history of thromboembolic events.
Data from the NSABP P-1 trial show that participants receiving tamoxifen without a history of pulmonary emboli (PE) had a statistically significant increase in pulmonary emboli (18- tamoxifen, 6-placebo, RR=3.01, 95% CI: 1.15-9.27). Three of the pulmonary emboli, all in the tamoxifen arm, were fatal. Eighty-seven percent of the cases of pulmonary embolism occurred in women at least 50 years of age at randomization. Among women receiving tamoxifen, the events appeared between 2 and 60 months (average = 27 months) from the start of treatment.
In this same population, a non-statistically significant increase in deep vein thrombosis (DVT) was seen in the tamoxifen group (30- tamoxifen, 19-placebo; RR=1.59, 95% CI: 0.86-2.98). The same increase in relative risk was seen in women ≤ 49 and in women ≥ 50, although fewer events occurred in younger women. Women with thromboembolic events were at risk for a second related event (7 out of 25 women on placebo, 5 out of 48 women on tamoxifen) and were at risk for complications of the event and its treatment (0/25 on placebo, 4/48 on tamoxifen). Among women receiving tamoxifen, deep vein thrombosis events occurred between 2 and 57 months (average = 19 months) from the start of treatment.
There was a non-statistically significant increase in stroke among patients randomized to tamoxifen (24-Placebo; 34- tamoxifen; RR=1.42; 95% CI: 0.82-2.51). Six of the 24 strokes in the placebo group were considered hemorrhagic in origin and 10 of the 34 strokes in the tamoxifen group were categorized as hemorrhagic. Seventeen of the 34 strokes in the tamoxifen group were considered occlusive and 7 were considered to be of unknown etiology. Fourteen of the 24 strokes on the placebo arm were reported to be occlusive and 4 of unknown etiology. Among these strokes 3 strokes in the placebo group and 4 strokes in the tamoxifen group were fatal. Eighty-eight percent of the strokes occurred in women at least 50 years of age at the time of randomization. Among women receiving tamoxifen, the events occurred between 1 and 63 months (average = 30 months) from the start of treatment.
Effects on the liver: Liver cancer: In the Swedish trial using adjuvant tamoxifen 40 mg/day for 2-5 years, 3 cases of liver cancer have been reported in the tamoxifen-treated group vs. 1 case in the observation group (See PRECAUTIONS -- Carcinogenesis). In other clinical trials evaluating tamoxifen, no cases of liver cancer have been reported to date.
One case of liver cancer was reported in NSABP P-1 in a participant randomized to tamoxifen.
Effects on the liver: Non-malignant effects: Tamoxifen has been associated with changes in liver enzyme levels, and on rare occasions, a spectrum of more severe liver abnormalities including fatty liver, cholestasis, hepatitis and hepatic necrosis. A few of these serious cases included fatalities. In most reported cases the relationship to tamoxifen is uncertain. However, some positive rechallenges and dechallenges have been reported.
In the NSABP P-1 trial, few grade 3-4 changes in liver function (SGOT, SGPT, bilirubin, alkaline phosphatase) were observed (10 on placebo and 6 on tamoxifen). Serum lipids were not systematically collected.
Other cancers: A number of second primary tumors, occurring at sites other than the endometrium, have been reported following the treatment of breast cancer with tamoxifen in clinical trials. Data from the NSABP B-14 and P-1 studies show no increase in other (non-uterine) cancers among patients receiving tamoxifen. Whether an increased risk for other (non-uterine) cancers is associated with tamoxifen is still uncertain and continues to be evaluated.
Effects on the Eye: Ocular disturbances, including corneal changes, decrement in color vision perception, retinal vein thrombosis, and retinopathy have been reported in patients receiving tamoxifen. An increased incidence of cataracts and the need for cataract surgery have been reported in patients receiving tamoxifen.
In the NSABP P-1 trial, an increased risk of borderline significance of developing cataracts among those women without cataracts at baseline (540- tamoxifen; 483-placebo; RR=1.13, 95% CI: 1.00-1.28) was observed. Among these same women, tamoxifen was associated with an increased risk of having cataract surgery (101-tamoxifen; 63-placebo; RR=1.62, 95% CI: 1.17-2.25) (See Table 3 in CLINICAL PHARMACOLOGY). Among all women on the trial (with or without cataracts at baseline), tamoxifen was associated with an increased risk of having cataract surgery (201- tamoxifen; 129-placebo; RR=1.51, 95% CI: 1.21-1.89). Eye examinations were not required during the study. No other conclusions regarding non-cataract ophthalmic events can be made.
Pregnancy Category D: Tamoxifen may cause fetal harm when administered to a pregnant woman. Women should be advised not to become pregnant while taking tamoxifen or within 2 months of discontinuing tamoxifen and should use barrier or nonhormonal contraceptive measures if sexually active. Tamoxifen does not cause infertility, even in the presence of menstrual irregularity. Effects on reproductive functions are expected from the antiestrogenic properties of the drug. In reproductive studies in rats at dose levels equal to or below the human dose, non-teratogenic developmental skeletal changes were seen and were found reversible. In addition, in fertility studies in rats and in teratology studies in rabbits using doses at or below those used in humans, a lower incidence of embryo implantation and a higher incidence of fetal death or retarded in utero growth were observed, with slower learning behavior in some rat pups when compared to historical controls. Several pregnant marmosets were dosed with 10 mg/kg/day (about 2-fold the daily maximum recommended human dose on a mg/m2 basis) during organogenesis or in the last half of pregnancy. No deformations were seen and, although the dose was high enough to terminate pregnancy in some animals, those that did maintain pregnancy showed no evidence of teratogenic malformations.
In rodent models of fetal reproductive tract development, tamoxifen (at doses 0.002 to 2.4-fold the daily maximum recommended human dose on a mg/m2 basis) caused changes in both sexes that are similar to those caused by estradiol, ethynylestradiol and diethylstilbestrol. Although the clinical relevance of these changes is unknown, some of these changes, especially vaginal adenosis, are similar to those seen in young women who were exposed to diethylstilbestrol in utero and who have a 1 in 1,000 risk of developing clear-cell adenocarcinoma of the vagina or cervix. To date, in utero exposure to tamoxifen has not been shown to cause vaginal adenosis, or clear-cell adenocarcinoma of the vagina or cervix, in young women. However, only a small number of young women have been exposed to tamoxifen in utero, and a smaller number have been followed long enough (to age 15-20) to determine whether vaginal or cervical neoplasia could occur as a result of this exposure.
There are no adequate and well-controlled trials of tamoxifen in pregnant women. There have been a small number of reports of vaginal bleeding, spontaneous abortions, birth defects, and fetal deaths in pregnant women. If this drug is used during pregnancy, or the patient becomes pregnant while taking this drug, or within approximately two months after discontinuing therapy, the patient should be apprised of the potential risks to the fetus including the potential long-term risk of a DES-like syndrome.
Reduction in Breast Cancer Incidence in High Risk Women--Pregnancy Category D: For sexually active women of child-bearing potential, tamoxifen therapy should be initiated during menstruation. In women with menstrual irregularity, a negative B-HCG immediately prior to the initiation of therapy is sufficient (See PRECAUTIONS -- Information for Patients -- Reduction in Breast Cancer Incidence in High Risk Women).
-
PRECAUTIONS
General: Decreases in platelet counts, usually to 50,000-100,000/mm3, infrequently lower, have been occasionally reported in patients taking tamoxifen for breast cancer. In patients with significant thrombocytopenia, rare hemorrhagic episodes have occurred, but it is uncertain if these episodes are due to tamoxifen therapy. Leukopenia has been observed, sometimes in association with anemia and/or thrombocytopenia. There have been rare reports of neutropenia and pancytopenia in patients receiving tamoxifen; this can sometimes be severe.
In the NSABP P-1 trial, 6 women on tamoxifen and 2 on placebo experienced grade 3-4 drops in platelet counts (≤50,000/mm3).
Information for Patients:
Reduction in Invasive Breast Cancer and DCIS in Women with DCIS: Women with DCIS treated with lumpectomy and radiation therapy who are considering tamoxifen to reduce the incidence of a second breast cancer event should assess the risks and benefits of therapy, since treatment with tamoxifen decreased the incidence of invasive breast cancer, but has not been shown to affect survival (See Table 1 in CLINICAL PHARMACOLOGY).
Reduction in Breast Cancer Incidence in High Risk Women: Women who are at high risk for breast cancer can consider taking tamoxifen therapy to reduce the incidence of breast cancer. Whether the benefits of treatment are considered to outweigh the risks depends on a woman's personal health history and on how she weighs the benefits and risks. Tamoxifen therapy to reduce the incidence of breast cancer may therefore not be appropriate for all women at high risk for breast cancer. Women who are considering tamoxifen therapy should consult their health care professional for an assessment of the potential benefits and risks prior to starting therapy for reduction in breast cancer incidence (See Table 3 in CLINICAL PHARMACOLOGY). Women should understand that tamoxifen reduces the incidence of breast cancer, but may not eliminate risk. Tamoxifen decreased the incidence of small estrogen receptor positive tumors, but did not alter the incidence of estrogen receptor negative tumors or larger tumors. In women with breast cancer who are at high risk of developing a second breast cancer, treatment with about 5 years of tamoxifen reduced the annual incidence rate of a second breast cancer by approximately 50%.
Women who are pregnant or who plan to become pregnant should not take tamoxifen to reduce her risk of breast cancer. Effective nonhormonal contraception must be used by all premenopausal women taking tamoxifen and for approximately two months after discontinuing therapy if they are sexually active. Tamoxifen does not cause infertility, even in the presence of menstrual irregularity. For sexually active women of child-bearing potential, tamoxifen therapy should be initiated during menstruation. In women with menstrual irregularity, a negative B-HCG immediately prior to the initiation of therapy is sufficient (See WARNINGS-Pregnancy Category D).
Two European trials of tamoxifen to reduce the risk of breast cancer were conducted and showed no difference in the number of breast cancer cases between the tamoxifen and placebo arms. These studies had trial designs that differed from that of NSABP P-1, were smaller than NSABP P-1, and enrolled women at a lower risk for breast cancer than those in P-1.
Monitoring During Tamoxifen Therapy: Women taking or having previously taken tamoxifen should be instructed to seek prompt medical attention for new breast lumps, vaginal bleeding, gynecologic symptoms (menstrual irregularities, changes in vaginal discharge, or pelvic pain or pressure), symptoms of leg swelling or tenderness, unexplained shortness of breath, or changes in vision. Women should inform all care providers, regardless of the reason for evaluation, that they take tamoxifen.
Women taking tamoxifen to reduce the incidence of breast cancer should have a breast examination, a mammogram, and a gynecologic examination prior to the initiation of therapy. These studies should be repeated at regular intervals while on therapy, in keeping with good medical practice. Women taking tamoxifen as adjuvant breast cancer therapy should follow the same monitoring procedures as for women taking tamoxifen for the reduction in the incidence of breast cancer. Women taking tamoxifen as treatment for metastatic breast cancer should review this monitoring plan with their care provider and select the appropriate modalities and schedule of evaluation.
Laboratory Tests: Periodic complete blood counts, including platelet counts, and periodic liver function tests should be obtained.
Drug Interactions: When tamoxifen is used in combination with coumarin-type anticoagulants, a significant increase in anticoagulant effect may occur. Where such coadministration exists, careful monitoring of the patient's prothrombin time is recommended.
In the NSABP P-1 trial, women who required coumarin-type anticoagulants for any reason were ineligible for participation in the trial (See CONTRAINDICATIONS).
There is an increased risk of thromboembolic events occurring when cytotoxic agents are used in combination with tamoxifen.
Tamoxifen reduced letrozole plasma concentrations by 37%. The effect of tamoxifen on metabolism and excretion of other antineoplastic drugs, such as cyclophosphamide and other drugs that require mixed function oxidases for activation, is not known. Tamoxifen and N-desmethyl tamoxifen plasma concentrations have been shown to be reduced when coadministered with rifampin or aminoglutethimide. Induction of CYP3A4-mediated metabolism is considered to be the mechanism by which these reductions occur; other CYP3A4 inducing agents have not been studied to confirm this effect.
One patient receiving tamoxifen with concomitant phenobarbital exhibited a steady state serum level of tamoxifen lower than that observed for other patients (ie, 26 ng/mL vs. mean value of 122 ng/mL). However, the clinical significance of this finding is not known. Rifampin induced the metabolism of tamoxifen and significantly reduced the plasma concentrations of tamoxifen in 10 patients. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
Concomitant bromocriptine therapy has been shown to elevate serum tamoxifen and N-desmethyl tamoxifen.
Drug/Laboratory Testing Interactions: During postmarketing surveillance, T4 elevations were reported for a few postmenopausal patients which may be explained by increases in thyroid-binding globulin. These elevations were not accompanied by clinical hyperthyroidism.
Variations in the karyopyknotic index on vaginal smears and various degrees of estrogen effect on Pap smears have been infrequently seen in postmenopausal patients given tamoxifen.
In the postmarketing experience with tamoxifen, infrequent cases of hyperlipidemias have been reported. Periodic monitoring of plasma triglycerides and cholesterol may be indicated in patients with pre-existing hyperlipidemias (See ADVERSE REACTIONS-Postmarketing experience section).
Carcinogenesis: A conventional carcinogenesis study in rats at doses of 5, 20, and 35 mg/kg/day (about one, three and seven-fold the daily maximum recommended human dose on a mg/m2 basis) administered by oral gavage for up to 2 years revealed a significant increase in hepatocellular carcinoma at all doses. The incidence of these tumors was significantly greater among rats administered 20 or 35 mg/kg/day (69%) compared to those administered 5 mg/kg/day (14%). In a separate study, rats were administered tamoxifen at 45 mg/kg/day (about nine-fold the daily maximum recommended human dose on a mg/m2 basis); hepatocellular neoplasia was exhibited at 3 to 6 months.
Granulosa cell ovarian tumors and interstitial cell testicular tumors were observed in two separate mouse studies. The mice were administered the trans and racemic forms of tamoxifen for 13 to 15 months at doses at 5, 20 and 50 mg/kg/day (about one-half, two and five-fold the daily recommended human dose on a mg/m2 basis).
Mutagenesis: No genotoxic potential was found in a conventional battery of in vivo and in vitro tests with pro- and eukaryotic test systems with drug metabolizing systems. However, increased levels of DNA adducts were observed by 32P post-labeling in DNA from rat liver and cultured human lymphocytes. Tamoxifen also has been found to increase levels of micronucleus formation in vitro in human lymphoblastoid cell line (MCL-5). Based on these findings, tamoxifen is genotoxic in rodent and human MCL-5 cells.
Impairment of Fertility: Tamoxifen produced impairment of fertility and conception in female rats at doses of 0.04 mg/kg/day (about 0.01-fold the daily maximum recommended human dose on a mg/m2 basis) when dosed for two weeks prior to mating through day 7 of pregnancy. At this dose, fertility and reproductive indices were markedly reduced with total fetal mortality. Fetal mortality was also increased at doses of 0.16 mg/kg/day (about 0.03-fold the daily maximum recommended human dose on a mg/m2 basis) when female rats were dosed from days 7-17 of pregnancy. Tamoxifen produced abortion, premature delivery and fetal death in rabbits administered doses equal to or greater than 0.125 mg/kg/day (about 0.05-fold the daily maximum recommended human dose on a mg/m2 basis). There were no teratogenic changes in either rats or rabbits.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from tamoxifen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: Approved labeling describing pediatric safety and efficacy information regarding tamoxifen use in patients with McCune-Albright syndrome is available for AstraZeneca's tamoxifen citrate tablets. However, due to AstraZeneca's marketing exclusivity rights, this drug product is not labeled for pediatric use. The long-term effects of tamoxifen therapy for girls have not been established. In adults treated with tamoxifen, an increase in incidence of endometrial adenocarcinoma and uterine sarcoma has been noted (see BOXED WARNING).
Geriatric Use: In the NSABP P-1 trial, the percentage of women at least 65 years of age was 16%. Women at least 70 years of age accounted for 6% of the participants. A reduction in breast cancer incidence was seen among participants in each of the subsets: A total of 28 and 10 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. Across all other outcomes, the results in this subset reflect the results observed in the subset of women at least 50 years of age. No overall differences in tolerability were observed between older and younger patients (See CLINICAL PHARMACOLOGY - Clinical Studies-Reduction in Breast Cancer Incidence in High Risk Women section).
In the NSABP B-24 trial, the percentage of women at least 65 years of age was 23%. Women at least 70 years of age accounted for 10% of participants. A total of 14 and 12 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. This subset is too small to reach any conclusions on efficacy. Across all other endpoints, the results in this subset were comparable to those of younger women enrolled in this trial. No overall differences in tolerability were observed between older and younger patients.
-
ADVERSE REACTIONS
Adverse reactions to tamoxifen are relatively mild and rarely severe enough to require discontinuation of treatment in breast cancer patients. Continued clinical studies have resulted in further information which better indicates the incidence of adverse reactions with tamoxifen as compared to placebo.
Metastatic Breast Cancer: Increased bone and tumor pain and, also, local disease flare have occurred, which are sometimes associated with a good tumor response. Patients with increased bone pain may require additional analgesics. Patients with soft tissue disease may have sudden increases in the size of preexisting lesions, sometimes associated with marked erythema within and surrounding the lesions and/or the development of new lesions. When they occur, the bone pain or disease flare are seen shortly after starting tamoxifen and generally subside rapidly.
In patients treated with tamoxifen for metastatic breast cancer, the most frequent adverse reaction to tamoxifen is hot flashes.
Other adverse reactions which are seen infrequently are hypercalcemia, peripheral edema, distaste for food, pruritus vulvae, depression, dizziness, light-headedness, headache, hair thinning and/or partial hair loss, and vaginal dryness.
Premenopausal Women: The following table summarizes the incidence of adverse reactions reported at a frequency of 2% or greater from clinical trials (Ingle, Pritchard, Buchanan) which compared tamoxifen therapy to ovarian ablation in premenopausal patients with metastatic breast cancer.
* Some women had more than one adverse reaction.
Tamoxifen Ovarian Tamoxifen Ovarian All Effects Ablation All Effects Ablation % of Women All Effects %of Women All Effects % of Women % of Women Adverse Reactions * n=104 n=100 Adverse Reactions * n=104 n=100 Flush 33 46 Edema 4 1 Amenorrhea 16 69 Fatigue 4 1 Altered Menses 13 5 Musculoskeletal Pain 3 0 Oligomenorrhea 9 1 Pain 3 4 Bone Pair 6 6 Ovarian Cyst(s) 3 2 Menstrual Disorder 6 4 Depression 2 2 Nausea 5 4 Abdominal Cramps 1 2 Cough/Coughing 4 1 Anorexia 1 2 Male Breast Cancer: Tamoxifen is well tolerated in males with breast cancer. Reports from the literature and case reports suggest that the safety profile of tamoxifen in males is similar to that seen in women. Loss of libido and impotence have resulted in discontinuation of tamoxifen therapy in male patients. Also, in oligospermic males treated with tamoxifen, LH, FSH, testosterone and estrogen levels were elevated. No significant clinical changes were reported.
Adjuvant Breast Cancer: In the NSABP B-14 study, women with axillary node-negative breast cancer were randomized to 5 years of tamoxifen 20 mg/day or placebo following primary surgery. The reported adverse effects are tabulated below (mean follow-up of approximately 6.8 years) showing adverse events more common on tamoxifen than on placebo. The incidence of hot flashes (64% vs. 48%), vaginal discharge (30% vs. 15%), and irregular menses (25% vs. 19%) were higher with tamoxifen compared with placebo. All other adverse effects occurred with similar frequency in the 2 treatment groups, with the exception of thrombotic events; a higher incidence was seen in tamoxifen-treated patients (through 5 years, 1.7% vs. 0.4%). Two of the patients treated with tamoxifen who had thrombotic events died.
*Defined as a platelet count of <100,000/mm3
NSABP B-14 Study % of Women % of Women Tamoxifen Placebo Tamoxifen Placebo Adverse Effect (N-1422) (N=1437) Adverse Effects (N=1422) (N=1437) Hot Flashes 64 48 Increased Bilirubin 2 1 Fluid Retention 32 30 Increased Creatinine 2 1 Vaginal Discharge 30 15 Thrombocytopenia * 2 1 Nausea 26 24 Thrombotic Events Irregular Menses 25 19 Deep Vein Thrombosis 0.8 0.2 Weight Loss (>5%) 23 18 Pulmonary Embolism 0.5 0.2 Skin Changes 19 15 Superficial Phlebitis 0.4 0.0 Increased SGOT 5 3 In the Eastern Cooperative Oncology Group (ECOG) adjuvant breast cancer trial, tamoxifen or placebo was administered for 2 years to women following mastectomy. When compared to placebo, tamoxifen showed a significantly higher incidence of hot flashes (19% vs. 8% for placebo). The incidence of all other adverse reactions was similar in the 2 treatment groups with the exception of thrombocytopenia where the incidence for tamoxifen was 10% vs. 3% for placebo, an observation of borderline statistical significance.
In other adjuvant studies, Toronto and Tamoxifen Adjuvant Trial Organization (NATO), women received either tamoxifen or no therapy. In the Toronto study, hot flashes were observed in 29% of patients for tamoxifen vs. 1% in the untreated group. In the NATO trial, hot flashes and vaginal bleeding were reported in 2.8% and 2.0% of women, respectively, for tamoxifen vs. 0.2% for each in the untreated group.
Ductal Carcinoma in Situ (DCIS): The type and frequency of adverse events in the NSABP B-24 trial were consistent with those observed in the other adjuvant trials conducted with tamoxifen.
Reduction in Breast Cancer Incidence in High Risk Women: In the NSABP P-1 Trial, there was an increase in five serious adverse effects in the tamoxifen group: endometrial cancer (33 cases in the tamoxifen group vs. 14 in the placebo group); pulmonary embolism (18 cases in the tamoxifen group vs. 6 in the placebo group); deep vein thrombosis (30 cases in the tamoxifen group vs. 19 in the placebo group); stroke (34 cases in the tamoxifen group vs. 24 in the placebo group); cataract formation (540 cases in the tamoxifen group vs. 483 in the placebo group) and cataract surgery (101 cases in the tamoxifen group vs. 63 in the placebo group) (See WARNINGS and Table 3 in CLINICAL PHARMACOLOGY).
The following table presents the adverse events observed in NSABP P-1 by treatment arm. Only adverse events more common on tamoxifen than placebo are shown.
1 Number with Quality of Life Questionnaires
2 Number with Treatment Follow-up Forms
3 Number with Adverse Drug Reaction Forms
NSABP P-1 Trial: All Adverse Events % of Women % of Women Tamoxifen Placebo Tamoxifen Placebo N=6681 N=6707 N=6681 N=6707 Self Reported Symptoms N=64411 N=64691 Adverse Effects N=64923 N=64843 Hot Flashes 80 68 Other Toxicities Vaginal Discharges 55 35 Mood 11.6 10.8 Vaginal Bleeding 23 22 Infection/Sepsis 6.0 5.1 Constipation 4.4 3.2 Laboratory Abnormalities N=65202 N=65352 Alopecia 5.2 4.4 Platelets decreased 0.7 0.3 Skin 5.6 4.7 Allergy 2.5 2.1 In the NSABP P-1 trial, 15.0% and 9.7% of participants receiving tamoxifen and placebo therapy, respectively withdrew from the trial for medical reasons. The following are the medical reasons for withdrawing from tamoxifen and placebo therapy, respectively: Hot flashes (3.1% vs. 1.5%) and Vaginal Discharge (0.5% vs. 0.1%).
In the NSABP P-1 Trial, 8.7% and 9.6% of participants receiving tamoxifen and placebo therapy, respectively withdrew for non-medical reasons.
On the NSABP P-1 Trial, hot flashes of any severity occurred in 68% of women on placebo and in 80% of women on tamoxifen. Severe hot flashes occurred in 28% of women on placebo and 45% of women on tamoxifen. Vaginal discharge occurred in 35% and 55% of women on placebo and tamoxifen respectively; and was severe in 4.5% and 12.3% respectively. There was no difference in the incidence of vaginal bleeding between treatment arms.
Postmarketing experience: Less frequently reported adverse reactions are vaginal bleeding, vaginal discharge, menstrual irregularities, skin rash and headaches. Usually these have not been of sufficient severity to require dosage reduction or discontinuation of treatment. Very rare reports of erythema multiforme, Stevens-Johnson syndrome, bullous pemphigoid, interstitial pneumonitis, and rare reports of hypersensitivity reactions including angioedema have been reported with tamoxifen therapy. In some of these cases, the time to onset was more than one year. Rarely, elevation of serum triglyceride levels, in some cases with pancreatitis, may be associated with the use of tamoxifen (see PRECAUTIONS -- Drug/Laboratory Testing Interactions section).
Pediatric Patients – McCune-Albright Syndrome: Approved labeling describing pediatric adverse reaction information regarding tamoxifen use in patients with McCune-Albright syndrome is available for AstraZeneca's tamoxifen citrate tablets. However, due to AstraZeneca's marketing exclusivity rights, this drug product is not labeled for pediatric use. The long-term effects of tamoxifen therapy for girls have not been established. Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted in adults treated with tamoxifen (see BOXED WARNING), continued monitoring of McCune-Albright patients treated with tamoxifen for long-term uterine effects is recommended.
-
OVERDOSAGE
Signs observed at the highest doses following studies to determine LD50 in animals were respiratory difficulties and convulsions.
Acute overdosage in humans has not been reported. In a study of advanced metastatic cancer patients which specifically determined the maximum tolerated dose of tamoxifen in evaluating the use of very high doses to reverse multidrug resistance, acute neurotoxicity manifested by tremor, hyperreflexia, unsteady gait and dizziness were noted. These symptoms occurred within 3-5 days of beginning tamoxifen and cleared within 2-5 days after stopping therapy. No permanent neurologic toxicity was noted. One patient experienced a seizure several days after tamoxifen was discontinued and neurotoxic symptoms had resolved. The causal relationship of the seizure to tamoxifen therapy is unknown. Doses given in these patients were all greater than 400 mg/m2 loading dose, followed by maintenance doses of 150 mg/m2 of tamoxifen given twice a day.
In the same study, prolongation of the QT interval on the electrocardiogram was noted when patients were given doses higher than 250 mg/m2 loading dose, followed by maintenance doses of 80 mg/m2 of tamoxifen given twice a day. For a woman with a body surface area of 1.5 m2 the minimal loading dose and maintenance doses given at which neurological symptoms and QT changes occurred were at least 6 fold higher in respect to the maximum recommended dose.
No specific treatment for overdosage is known; treatment must be symptomatic.
-
DOSAGE AND ADMINISTRATION
For patients with breast cancer, the recommended daily dose is 20-40 mg. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
In three single agent adjuvant studies in women, one 10 mg tamoxifen citrate tablet was administered two (ECOG and NATO) or three (Toronto) times a day for two years. In the NSABP B-14 adjuvant study in women with node-negative breast cancer, one 10 mg tamoxifen citrate tablet was given twice a day for at least five years. Results of the B-14 study suggest that continuation of therapy beyond five years does not provide additional benefit (see CLINICAL PHARMACOLOGY). In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy. There was no indication that doses greater than 20 mg per day were more effective. Current data from clinical trials support five years of adjuvant tamoxifen therapy for patients with breast cancer.
Ductal Carcinoma in Situ (DCIS): The recommended dose is tamoxifen citrate tablet 20 mg daily for 5 years.
Reduction in Breast Cancer Incidence in High Risk Women: The recommended dose is tamoxifen citrate tablets 20 mg daily for 5 years. There are no data to support the use of tamoxifen other than for 5 years (See CLINICAL PHARMACOLOGY -- Clinical Studies--Reduction in Breast Cancer Incidence in High Risk Women).
-
HOW SUPPLIED
10 mg Tablets: Tamoxifen Citrate Tablets, USP are available as 10 mg tablets (containing 15.2 mg tamoxifen citrate in an amount equivalent to 10 mg of tamoxifen) round, biconvex, uncoated, white tablet unscored identified with "964" debossed on one side and the
 debossed on the other side are supplied in bottles of 60 tablets (NDC: 62037-964-60), 180 tablets (NDC: 62037-964-18) and 2500 tablets (NDC: 62037-964-25).
debossed on the other side are supplied in bottles of 60 tablets (NDC: 62037-964-60), 180 tablets (NDC: 62037-964-18) and 2500 tablets (NDC: 62037-964-25).20 mg Tablets: Tamoxifen Citrate Tablets, USP are available as 20 mg tablets (containing 30.4 mg tamoxifen citrate in an amount equivalent to 20 mg of tamoxifen) round, biconvex, uncoated, white tablet unscored identified with "965" debossed on one side and the
 debossed on the other side are supplied in bottles of 30 tablets (NDC: 62037-965-30), 90 tablets (NDC: 62037-965-90) and 1250 tablets (NDC: 62037-965-12).
debossed on the other side are supplied in bottles of 30 tablets (NDC: 62037-965-30), 90 tablets (NDC: 62037-965-90) and 1250 tablets (NDC: 62037-965-12).Store at controlled room temperature, 20-25°C (68-77°F) [see USP]. Dispense in a well-closed, light-resistant container.
Manufactured by:
Andrx Pharmaceuticals, Inc.
Ft. Lauderdale, FL 33314
Toll Free 1-866-292-6719Rev. date: 02/03A
7227 -
SUPPLEMENTAL PATIENT MATERIAL
PATIENT INFORMATION ABOUT
TAMOXIFEN CITRATE TABLETS
for Breast Cancer Treatment and Reduction in the Incidence of Breast Cancer
Generic Name: Tamoxifen (ta-MOX-i-fen)
Please read this information carefully before you begin taking tamoxifen. It is important to read this information each time your prescription is filled or refilled in case new information is available. This summary does not tell you everything about tamoxifen. Your health care professional is the best source of information about this medicine. You should talk with him or her before you begin taking tamoxifen and at regular check-ups. In addition, the professional package insert contains more detailed information on tamoxifen.
What are the most important things I should know about tamoxifen citrate tablets?
Tamoxifen has been shown to help women with advanced breast cancer and in clinical trials of over 30,000 women with early breast cancer it has been shown to reduce the risk of recurrence. Also in a trial of 13,000 women at high risk of breast cancer, tamoxifen reduced the risk of developing the disease.
Like all medicines, tamoxifen has some side effects. Most are mild and relate to its hormonal mode of action. For all women tamoxifen can, however, also increase the risk of some serious and potentially life-threatening events, including, uterine cancer, blood clots, and stroke. Some of these events have caused death. Tamoxifen can also increase the risk of getting cataracts or of needing cataract surgery. If you experience symptoms of any of these, tell you doctor immediately (see "What should I avoid or do while taking tamoxifen?").
If you are a woman at high risk for breast cancer or a woman with DCIS considering tamoxifen to reduce your risk of developing breast cancer, you should discuss the potential benefits versus the potential risks of these serious events with your health care provider.
What is tamoxifen?
- tamoxifen is a prescription medicine used to reduce the risk of getting breast cancer (in women who have a high risk of getting breast cancer).
This effect was shown in the Breast Cancer Prevention Trial (BCPT, NSABP P-1), a large study where over 13,000 women at high risk for breast cancer were to take tamoxifen or placebo (a pill without tamoxifen) for 5 years. High risk women were those who were at least 35 years old and had a combination of risks that made their chances of developing breast cancer greater than 1.67% in the next 5 years. The risk factors included early age at first menstrual period, late age at first pregnancy, no pregnancies, close family members with breast cancer (mother, sister, or daughter), history of previous breast biopsies, or high-risk changes in the breast seen on a biopsy. Twenty-five percent of the women in the study completed 5 years of treatment, and most women in this study have been followed for about 4 years. The study showed that tamoxifen reduced the chance of getting breast cancer by 44%. The longer-term effects of tamoxifen on reducing the chance of getting breast cancer are not known.
We do not know whether taking tamoxifen for 5 years only delays the appearance of cancer, or actually decreases the number of tumors that will ever develop since long-term studies have not been completed.
Some women in this study also experienced serious side effects of tamoxifen. They are described in detail in the section, What are the possible side effects of tamoxifen?. Some of these women experienced complications related to the treatment of these side effects. The following table of the major results from the study is intended to be an aid in weighing the potential benefit of a reduction in risk of breast cancer against the potential risk of serious side effects of tamoxifen.
* In women with a uterus.
Cases per year out of
1000 Women taking
TamoxifenCases per year out of
1000 Women taking
PlaceboBreast Cancer 3.6 6.5 Endometrial Cancer * 2.3 0.9 Blood clot in the lungs 0.8 0.3 Blood clot in the veins 1.3 0.8 Stroke 1.4 1.0 Cataracts 25.4 22.5 Cataract surgery 46.6 31.4 Two European trials of tamoxifen in women with a high risk of breast cancer were also conducted. They showed no difference in the number of breast cancer cases between the women who took tamoxifen and those who got placebo. These studies had trial designs that differed from that of NSABP P-1, were smaller than P-1, and enrolled women at a lower risk for breast cancer than those in the P-1 trial.
- In women with DCIS, following breast surgery and radiation, tamoxifen is indicated to reduce the risk of invasive breast cancer. The decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy.
A trial evaluated the addition of tamoxifen to lumpectomy and radiation therapy in women with DCIS. The primary objective was to determine whether 5 years of tamoxifen therapy would reduce the incidence of invasive breast cancer in the ipsilateral (the same) or contralateral (the opposite) breast. The incidence of invasive breast cancer was reduced by 43% among women treated with tamoxifen.
- Tamoxifen is used to reduce the recurrence of breast cancer in women who have had surgery and/or radiation therapy to treat early breast cancer. Tamoxifen is also used in women with breast cancer who are at risk of developing a second breast cancer in the opposite breast.
The Early Breast Cancer Trialists Collaborative Group reviewed the 10-year results of studies of tamoxifen for early breast cancer. Treatment with tamoxifen for about 5 years reduced the risk of recurrence of breast cancer and improved overall survival. Treatment with about 5 years of tamoxifen also reduced the chance of getting a second breast cancer in the opposite breast by approximately 50%, a result similar to that seen in the NSABP P-1 study.
- Tamoxifen is used to treat advanced breast cancer in women and men.
Three studies compared tamoxifen to surgery or radiation to the ovaries in premenopausal women with advanced breast cancer and found that tamoxifen was similar to surgery or radiation in causing tumor shrinkage.
Published studies have demonstrated that tamoxifen is effective for the treatment of advanced breast cancer in men.
- Tamoxifen is a prescription tablet available in two dosage strengths: 10 mg tablets and 20 mg tablets. The active ingredient in each tablet is tamoxifen citrate.
How does tamoxifen work?
Tamoxifen belongs to a group of medicines called antiestrogens. Antiestrogens work by blocking the effects of the hormone estrogen in the body. Estrogen may cause the growth of some types of breast tumors. Tamoxifen may block the growth of tumors that respond to estrogen.
Who should not take tamoxifen citrate tablets?
- You should not take tamoxifen to reduce the risk of getting breast cancer if you have ever had blood clots or if you develop blood clots that require medical treatment. However, if you are taking tamoxifen for treatment of early or advanced breast cancer, the benefits of tamoxifen may outweigh the risks associated with developing new blood clots. Your health care professional can assist you in deciding whether tamoxifen is right for you.
- You should not take tamoxifen to reduce the risk of getting breast cancer if you are taking medicines to thin your blood (anticoagulants) like warfarin (Coumadin®*).
- You should not take tamoxifen if you plan to become pregnant while taking tamoxifen or during the two months after you stop taking it because tamoxifen may harm your unborn child. You should see your doctor immediately and stop taking tamoxifen if you become pregnant while taking the drug. Please talk with your doctor about birth control recommendations. If you are capable of becoming pregnant, you should start tamoxifen during a menstrual period or if you have irregular periods have a negative pregnancy test before beginning to take tamoxifen. Tamoxifen does not prevent pregnancy, even in the presence of menstrual irregularity.
- You should not take tamoxifen if you are breast feeding.
- You should not take tamoxifen if you have ever had an allergic reaction to tamoxifen or tamoxifen citrate (the chemical name) or any of its ingredients.
- Tamoxifen is not known to reduce the risk of breast cancer in women with changes in breast cancer genes (BRCA1 or BRCA2).
- You should not take tamoxifen to decrease the chance of getting breast cancer if you are less than age 35 because tamoxifen has not been tested in younger women.
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of getting breast cancer. Certain conditions put women at high risk and it is possible to calculate this risk for any woman. Breast cancer risk assessment tools to help calculate your risk of breast cancer have been developed and are available to your health care professional. You should discuss your risks with your health care professional.
Girls with McCune-Albright Syndrome (a genetic condition associated with premature puberty) under the age of two and older than 10 years of age should not take tamoxifen because treatment in this age group has not been studied. Tamoxifen has not been studied in boys.
How should I take tamoxifen?
- Follow your doctor's instructions about when and how to take tamoxifen. Read the label on the container. If you are unsure or have questions, ask your doctor or pharmacist.
- You will take tamoxifen differently, depending on your diagnosis.
- For reduction of the risk of breast cancer, the usual dose is 20 mg a day, for five years.
- For treatment of breast cancer in adult women and men, the usual dose is 20-40 mg a day. Take the tablets once or twice a day depending on the tablet strength prescribed. If your doctor has prescribed a different dose, do not change it unless he or she tells you to do so. For women with early breast cancer, tamoxifen should be taken for 5 years. For women with advanced cancer, tamoxifen should be taken until your doctor feels it is no longer indicated.
- Take your medicine each day. You may find it easier to remember to take your medicine if you take it at the same time each day. If you forget to take a dose, take it as soon as you remember and then take the next dose as usual.
- Swallow the tablets whole with a drink of water.
- You can take tamoxifen with or without food.
- Do not stop taking your tablets unless your doctor tells you to do so.
Are there other important factors to consider before taking tamoxifen?
- Tell your doctor if you have ever had blood clots that required medical treatment.
- Because tamoxifen may affect how other medicines work, always tell your doctor if you are taking any other prescription or non-prescription (over-the-counter) medications, particularly if you are taking warfarin to thin your blood.
- You should not become pregnant when taking tamoxifen or during the two months after you stop taking it as tamoxifen may harm your unborn child. Please contact your doctor for birth control recommendations. Tamoxifen does not prevent pregnancy, even in the presence of menstrual irregularity. You should see your doctor immediately if you think you may have become pregnant after starting to take tamoxifen.
What should I avoid or do while taking tamoxifen?
- You should contact your doctor immediately if you notice any of the following symptoms. Some of these symptoms may suggest that you are experiencing a rare but serious side effect associated with tamoxifen (see "What are the possible side effects of tamoxifen?").
- – new breast lumps
- – vaginal bleeding
- – changes in your menstrual cycle
- – changes in vaginal discharge
- – pelvic pain or pressure
- – swelling or tenderness in your calf
- – unexplained breathlessness (shortness of breath)
- – sudden chest pain
- – coughing up blood
- – changes in your vision
- Because tamoxifen may affect how other medicines work, always tell your doctor if you are taking any other prescription or non-prescription (over-the-counter) medicines. Be sure to tell your doctor if you are taking warfarin (Coumadin) to thin your blood.
- You should not become pregnant when taking tamoxifen or during the two months after you stop taking it because tamoxifen may harm your unborn child. You should see your doctor immediately if you think you may have become pregnant after starting to take tamoxifen. Please talk with your doctor about birth control recommendations. If you are taking tamoxifen to reduce your risk of getting breast cancer, and you are sexually active, tamoxifen should be started during your menstrual period. If you have irregular periods, you should have a negative pregnancy test before you start tamoxifen. Tamoxifen does not prevent pregnancy, even in the presence of menstrual irregularity.
- If you are taking tamoxifen to reduce your risk of getting breast cancer, you should know that tamoxifen does not prevent all breast cancers. While you are taking tamoxifen and after you stop taking tamoxifen and in keeping with your doctor's recommendation, you should have annual gynecological check-ups which should include breast exams and mammograms. If breast cancer occurs, there is no guarantee that it will be detected at an early stage. This is why it is important to continue with regular check-ups.
What are the possible side effects of tamoxifen?
Like many medicines, tamoxifen causes side effects in most patients. The majority of the side effects seen with tamoxifen have been mild and do not usually cause breast cancer patients to stop taking the medication. In women with breast cancer, withdrawal from tamoxifen therapy is about 5%. Approximately 15% of women who took tamoxifen to reduce the chance of getting breast cancer stopped treatment because of side effects.
The most common side effects reported with tamoxifen are: hot flashes; vaginal discharge or bleeding; and menstrual irregularities (these side effects may be mild or may be a sign of a more serious side effect). Women may experience hair loss, skin rashes (itching or peeling skin) or headaches; or inflammation of the lungs, which may have the same symptoms as pneumonia, such as breathlessness and cough; however, hair loss is uncommon and is usually mild.
A rare but serious side effect of tamoxifen is a blood clot in the veins. Blood clots stop the flow of blood and can cause serious medical problems, disability, or death. Women who take tamoxifen are at increased risk for developing blood clots in the lungs and legs. Some women may develop more than one blood clot, even if tamoxifen is stopped. Women may also have complications from treating the clot, such as bleeding from thinning the blood too much. Symptoms of a blood clot in the lungs may include sudden chest pain, shortness of breath or coughing up blood. Symptoms of a blood clot in the legs are pain or swelling in the calves. A blood clot in the legs may move to the lungs. If you experience any of these symptoms of a blood clot, contact your doctor immediately.
Tamoxifen increases the chance of having a stroke, which can cause serious medical problems, disability, or death. If you experience any symptoms of stroke, such as weakness, difficulty walking or talking, or numbness, contact your doctor immediately.
Tamoxifen increases the chance of changes occurring in the lining (endometrium) or body of your uterus, which can be serious and could include cancer. If you have not had a hysterectomy (removal of the uterus), it is important for you to contact your doctor immediately if you experience any unusual vaginal discharge, vaginal bleeding, or menstrual irregularities; or pain or pressure in the pelvis (lower stomach). These may be caused by changes to the lining (endometrium) or body of your uterus. It is important to bring them to your doctor's attention without delay as they can occasionally indicate the start of something more serious and even life-threatening.
Tamoxifen may cause cataracts or changes to parts of the eye known as the cornea or retina. Tamoxifen can increase the chance of needing cataract surgery, and can cause blood clots in the veins of the eye. Tamoxifen can result in difficulty in distinguishing different colors. If you experience any changes in your vision, tell your doctor immediately.
Rare side effects, which may be serious, include certain liver problems such as jaundice (which may be seen as yellowing of the whites of the eyes) or hypertriglyceridemia (increased levels of fats in the blood) sometimes with pancreatitis (pain or tenderness in the upper abdomen). Stop taking tamoxifen and contact your doctor immediately if you develop angioedema (swelling of the face, lips, tongue and/or throat) even if you have been taking tamoxifen for a long time.
If you are a woman receiving tamoxifen for treatment of advanced breast cancer, and you experience excessive nausea, vomiting or thirst, tell your doctor immediately. This may mean that there are changes in the amount of calcium in your blood (hypercalcemia). Your doctor will evaluate this.
In patients with breast cancer, a temporary increase in the size of the tumor may occur and sometimes results in muscle aches/bone pain and skin redness. This condition may occur shortly after starting tamoxifen and may be associated with a good response to treatment.
Many of these side effects happen only rarely. However, you should contact your doctor if you think you have any of these or any other problems with your tamoxifen. Some side effects of tamoxifen may become apparent soon after starting the drug, but others may first appear at any time during therapy.
This summary does not include all possible side effects with tamoxifen. It is important to talk to your health care professional about possible side effects. If you want to read more, ask your doctor or pharmacist to give you the professional labeling.
How should I store tamoxifen?
Tamoxifen citrate tablets should be stored at room temperature (68-77°F). Keep in a well-closed, light-resistant container. Keep out of the reach of children.
Do not take your tablets after the expiration date on the container. Be sure that any discarded tablets are out of the reach of children.
This leaflet provides you with a summary of information about tamoxifen. Medicines are sometimes prescribed for uses other than those listed. Tamoxifen has been prescribed specifically for you by your doctor. Do not give your medicine to anyone else, even if they have a similar condition, because it may harm them.
If you have any questions or concerns, contact your doctor or pharmacist. Your pharmacist also has a longer leaflet about tamoxifen written for health care professionals that you can ask to read. For more information about tamoxifen or breast cancer, call 1-866-292-6719.
*Coumadin® is a registered trademark of Bristol-Myers Squibb Pharmaceuticals.
Manufactured by:
Andrx Pharmaceuticals, Inc.
Ft. Lauderdale, FL 33314 - tamoxifen is a prescription medicine used to reduce the risk of getting breast cancer (in women who have a high risk of getting breast cancer).
-
INGREDIENTS AND APPEARANCE
TAMOXIFEN CITRATE
tamoxifen citrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62037-964 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tamoxifen Citrate (UNII: 7FRV7310N6) (Tamoxifen - UNII:094ZI81Y45) 10 mg Inactive Ingredients Ingredient Name Strength carboxymethylcellulose calcium () magnesium stearate (UNII: 70097M6I30) mannitol (UNII: 3OWL53L36A) starch () Product Characteristics Color WHITE (WHITE) Score no score Shape ROUND (ROUND) Size 8mm Flavor Imprint Code 964 Contains Coating false Symbol true Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62037-964-60 60 in 1 BOTTLE, PLASTIC 2 NDC: 62037-964-18 180 in 1 BOTTLE, PLASTIC 3 NDC: 62037-964-25 2500 in 1 BOTTLE, PLASTIC TAMOXIFEN CITRATE
tamoxifen citrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62037-965 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tamoxifen Citrate (UNII: 7FRV7310N6) (Tamoxifen - UNII:094ZI81Y45) 20 mg Inactive Ingredients Ingredient Name Strength carboxymethylcellulose calcium () magnesium stearate (UNII: 70097M6I30) mannitol (UNII: 3OWL53L36A) starch () Product Characteristics Color WHITE (WHITE) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 965 Contains Coating false Symbol true Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62037-965-30 30 in 1 BOTTLE, PLASTIC 2 NDC: 62037-965-90 90 in 1 BOTTLE, PLASTIC 3 NDC: 62037-965-12 1250 in 1 BOTTLE, PLASTIC Labeler - Andrx Pharmaceuticals, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.