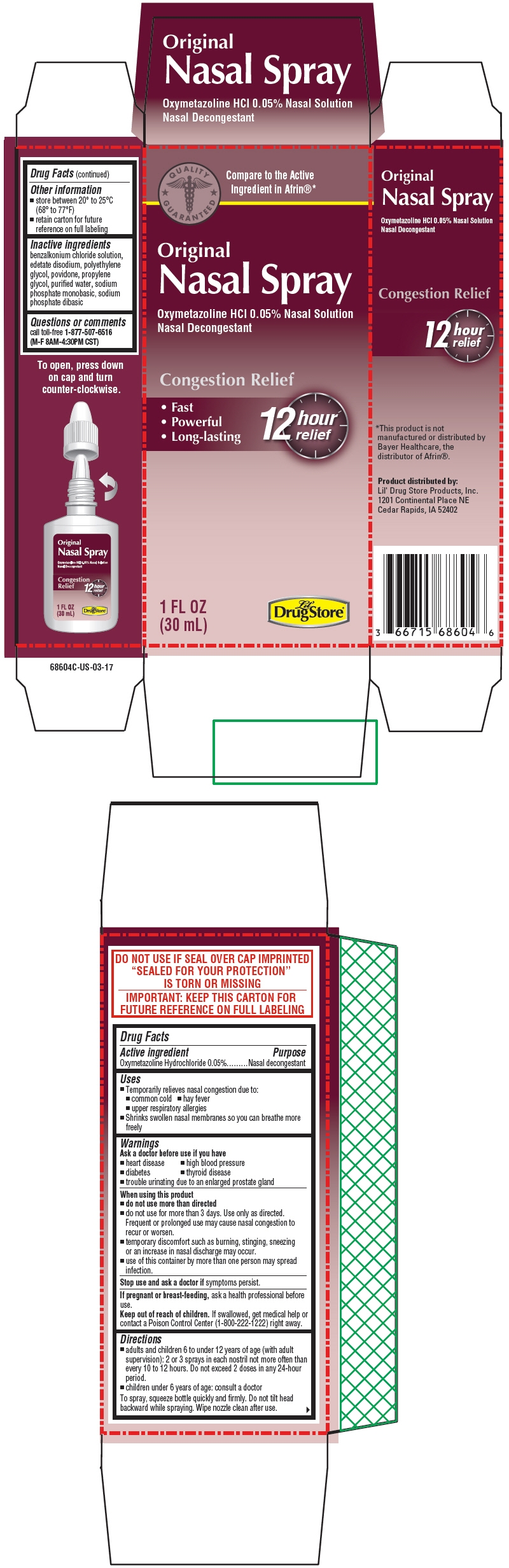
CONGESTION RELIEF LIL DRUG STORE PRODUCTS- oxymetazoline hydrochloride spray
Congestion Relief by
Drug Labeling and Warnings
Congestion Relief by is a Otc medication manufactured, distributed, or labeled by Lil' Drug Store Products, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur.
- use of this container by more than one person may spread infection.
-
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: consult a doctor
To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use.
- Other information
- Inactive ingredients
- Questions or comments
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
CONGESTION RELIEF LIL DRUG STORE PRODUCTS
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 66715-6860 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxymetazoline Hydrochloride (UNII: K89MJ0S5VY) (Oxymetazoline - UNII:8VLN5B44ZY) Oxymetazoline Hydrochloride 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Edetate Disodium (UNII: 7FLD91C86K) Glycerin (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66715-6860-4 1 in 1 CARTON 12/15/2019 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/01/2014 Labeler - Lil' Drug Store Products, Inc (093103646)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.