Soft & Silky/Health Guard Antibacterial Hand
Soft and Silky/Health Guard Antibacterial Hand by
Drug Labeling and Warnings
Soft and Silky/Health Guard Antibacterial Hand by is a Otc medication manufactured, distributed, or labeled by Kutol Products Company, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
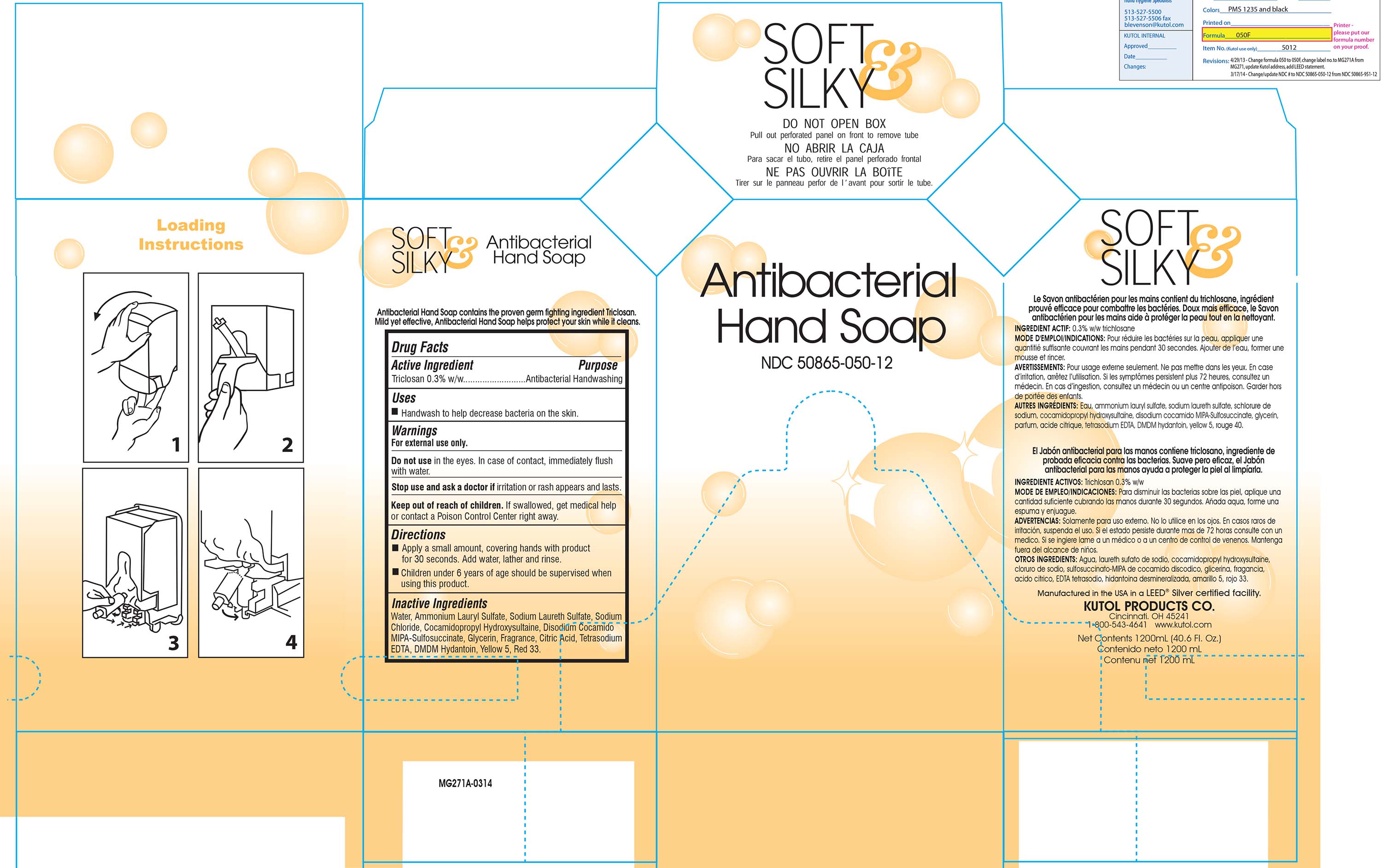
SOFT AND SILKY/HEALTH GUARD ANTIBACTERIAL HAND- antibacterial hand soap liquid
Kutol Products Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Soft & Silky/Health Guard Antibacterial Hand
Water, Ammounium Lauryl Sulfate, Sodium Laureth Sulfate, Sodium Chloride, Cocamidopropyl Hydroxysultaine, Disodium Cocamido MIPA-Sulfosuccinate, Glycerin, Fragrance, Citric Acid, Tetrasodium EDTA, DMDM Hydantoin, Yellow 5, Red 33.
50865-050-12/65/90: Handwash to help decrease bacteria on the skin.
50865-050-19/67/82/93: For handwashing to decrease bacteria on skin.
50865-050-09/12/65/82:
For external use only.
Do not use in the eyes. In case of contact, immediately flush with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
50865-050-19/67/93:
For external use only.
Do not use in the eyes.
Discontinue/Stop use if, in rare instances, irritation and redness develop. If condition persists for more than 72 hours, consult a physician.
Keep out of reach of children. If swallowed, contact a physician or poison control center.
50865-050-09/12/65:
- Apply a small amount, covering hands with product for 30 seconds. Add water, lather and rinse.
- Children under six years of age should be supervised when using this product.
50865-050-82/93:
To decrease bacteria on skin, apply a small amount, covering hands with product for 30 seconds. Add water, later and rinse.
50865-050-67: Wet hands, apply a small amount of product and work into a lather for 20 seconds, rinse thoroughly, dry hands completely.
50865-050-19: Apply a small amount, covering hands with product for 30 seconds. Add water, lather and rinse.
| SOFT AND SILKY/HEALTH GUARD ANTIBACTERIAL HAND
antibacterial hand soap liquid |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kutol Products Company, Inc. (004236139) |
| Registrant - Kutol Products Company, Inc. (004236139) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kutol Products Company, Inc. | 004236139 | manufacture(50865-050) , pack(50865-050) , label(50865-050) , analysis(50865-050) | |
 50865-050-82.jpg
50865-050-82.jpg
 50865-050-12.jpg
50865-050-12.jpg