ULINE ANTACID- calcium carbonate tablet, chewable
Uline Antacid by
Drug Labeling and Warnings
Uline Antacid by is a Otc medication manufactured, distributed, or labeled by Uline, Unifirst First Aid Corporation, Prestige Packaging, Medique Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
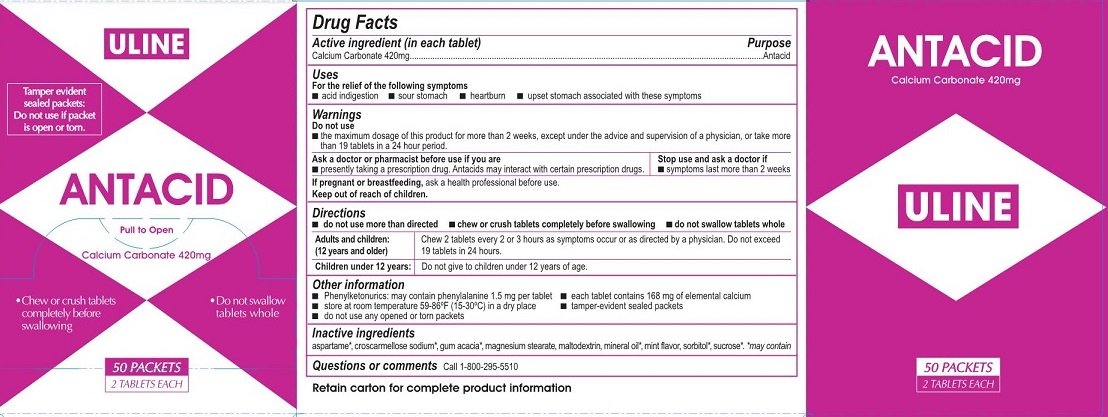
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use
- the maximum dosage of this product for more than 2 weeks except under the advice and supervision of a
physician or take more than 19 tablets in a 24 hour period.
- If pregnant or breast feeding,
- Keep out of the reach of children.
-
Directions
- do not use more than directed
- chew or crush tablets completely before swallowing
- do not swallow tablets whole
Adults and children: (12 years and older) Chew 2 tablets every 2 or 3 hours as symptoms occur or as directed by a physician. Do notexceed 19 tablets in 24 hours.
Children under 12 years: Do not give to children under 12 years of age.
- Other information
- Inactive ingredients
- Questions or comments?
- Uline Antacid Label
- Uline Antacid Label
-
INGREDIENTS AND APPEARANCE
ULINE ANTACID
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69790-089(NDC:47682-089) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) ACACIA (UNII: 5C5403N26O) SUCROSE (UNII: C151H8M554) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor MINT Imprint Code FR;8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69790-089-33 50 in 1 BOX 10/07/2019 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 10/07/2019 ULINE ANTACID
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69790-820(NDC:47682-820) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SORBITOL (UNII: 506T60A25R) MINERAL OIL (UNII: T5L8T28FGP) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;036 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69790-820-33 50 in 1 BOX 10/07/2019 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 10/07/2019 Labeler - Uline (039612668) Registrant - Unifirst First Aid Corporation (832947092) Establishment Name Address ID/FEI Business Operations Prestige Packaging 170837962 relabel(69790-089, 69790-820) , repack(69790-089, 69790-820)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.