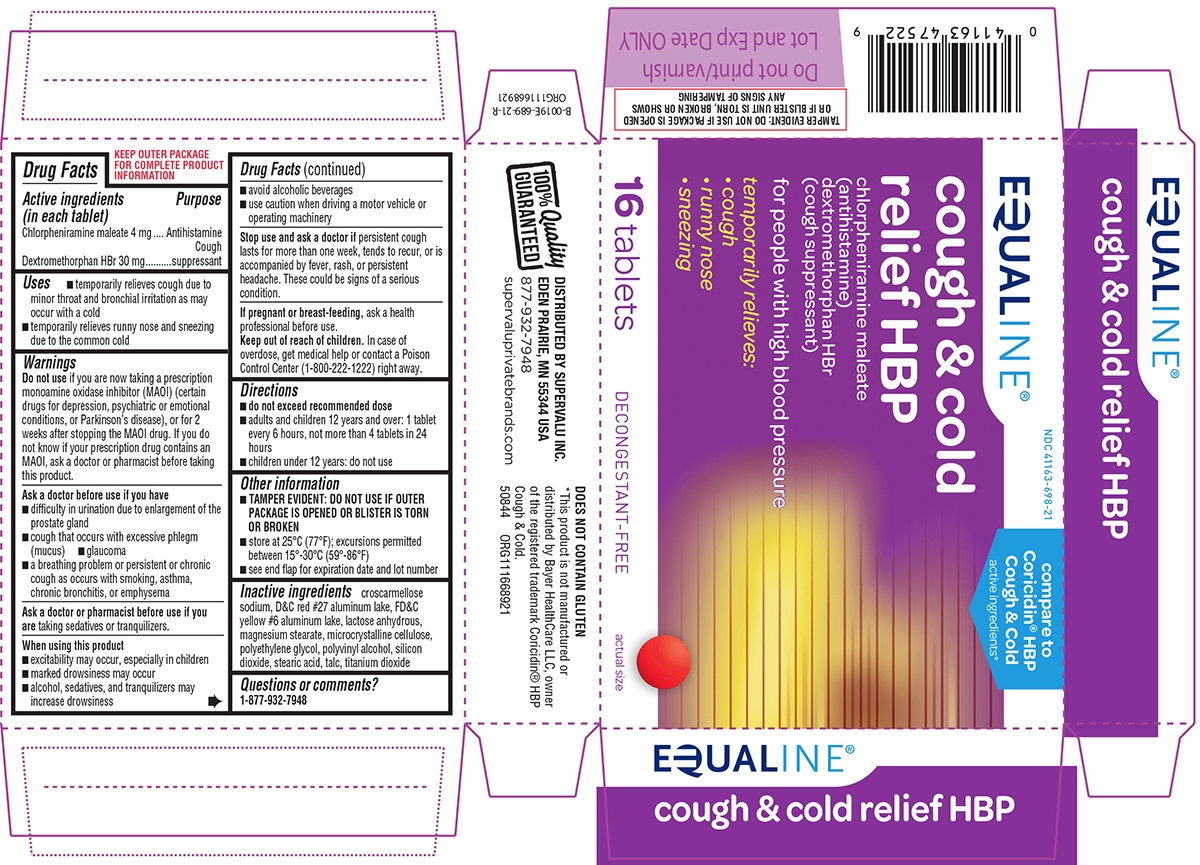
COUGH AND COLD RELIEF HBP- chlorpheniramine maleate, dextromethorphan hbr tablet, coated
cough and cold relief HBP by
Drug Labeling and Warnings
cough and cold relief HBP by is a Otc medication manufactured, distributed, or labeled by United Natural Foods, Inc. dba UNFI, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- cough that occurs with excessive phlegm (mucus)
- glaucoma
- a breathing problem or persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
EQUALINE®
NDC: 41163-698-21
compare to
Coricidin® HBP
Cough & Cold
active ingredients*cough & cold
relief HBPchlorpheniramine maleate
(antihistamine)
dextromethorphan HBr
(cough suppressant)for people with high blood pressure
temporarily relieves:
cough
runny nose
sneezing16 tablets
DECONGESTANT-FREE
actual size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
100% Quality
GUARANTEEDDISTRIBUTED BY SUPERVALU INC.
EDEN PRAIRIE, MN 55344 USA877-932-7948
supervaluprivatebrands.comDOES NOT CONTAIN GLUTEN
*This product is not manufactured or distributed
by Bayer HealthCare LLC, owner of the
registered trademark Coricidin® HBP Cough & Cold.
50844 ORG111668921
Equaline 44-689
-
INGREDIENTS AND APPEARANCE
COUGH AND COLD RELIEF HBP
chlorpheniramine maleate, dextromethorphan hbr tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41163-698 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Product Characteristics Color RED Score no score Shape ROUND Size 9mm Flavor Imprint Code 44;689 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41163-698-21 2 in 1 CARTON 03/31/2017 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 03/31/2017 Labeler - SUPERVALU INC. (006961411) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(41163-698) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(41163-698)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.