PHENOBARBITAL SODIUM injection
Phenobarbital Sodium by
Drug Labeling and Warnings
Phenobarbital Sodium by is a Prescription medication manufactured, distributed, or labeled by Cameron Pharmaceuticals, Vitae Enim Vitae Scientific, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The barbiturates are nonselective central nervous system (CNS) depressants which are primarily used as sedative hypnotics and also anticonvulsants in subhypnotic doses. The barbiturates and their sodium salts are subject to control under the Federal Controlled Substances Act (CIV).
Barbiturates are substituted pyrimidine derivatives in which the basic structure common to these drugs is barbituric acid, a substance which has no central nervous system activity. CNS activity is obtained by substituting alkyl, alkenyl or aryl groups on the pyrimidine ring.
Phenobarbital Sodium Injection, USP is a sterile solution for intramuscular or slow intravenous administration as a long-acting barbiturate. Each mL contains phenobarbital sodium either 65 mg or 130 mg, alcohol 0.1 mL, propylene glycol 0.678 mL and benzyl alcohol 0.015 mL in Water for Injection; hydrochloric acid added, if needed, for pH adjustment. The pH range is 9.2-10.2.
Chemically, phenobarbital sodium is 2,4,6(1H,3H,5H)-Pyrimidinetrione,5-ethyl-5-phenyl-, monosodium salt and has the following structural formula:
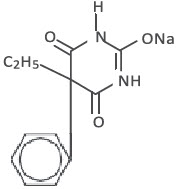
C12 H 11 N 2 NaO 3 MW 254.22
The sodium salt of phenobarbital occurs as a white, slightly bitter powder, crystalline granules or flaky crystals; it is soluble in alcohol and practically insoluble in ether or chloroform.
-
CLINICAL PHARMACOLOGY
Barbiturates are capable of producing all levels of CNS mood alteration from excitation to mild sedation, to hypnosis, and deep coma. Overdosage can produce death. In high enough therapeutic doses, barbiturates induce anesthesia. Barbiturates depress the sensory cortex, decrease motor activity, alter cerebellar function and produce drowsiness, sedation and hypnosis.
Barbiturate-induced sleep differs from physiological sleep. Sleep laboratory studies have demonstrated that barbiturates reduce the amount of time spent in the rapid eye movement (REM) phase of sleep or dreaming stage. Also, Stages III and IV sleep are decreased. Following abrupt cessation of barbiturates used regularly, patients may experience markedly increased dreaming, nightmares and/or insomnia. Therefore, withdrawal of a single therapeutic dose over 5 or 6 days has been recommended to lessen the REM rebound and disturbed sleep which contribute to drug withdrawal syndrome (for example, decrease the dose from 3 to 2 doses a day for 1 week).
In studies, secobarbital sodium and pentobarbital sodium have been found to lose most of their effectiveness for both inducing and maintaining sleep by the end of 2 weeks of continued drug administration even with the use of multiple doses. The short-, intermediate- and to a lesser degree, long-acting barbiturates have been widely prescribed for treating insomnia. Although the clinical literature abounds with claims that the short-acting barbiturates are superior for producing sleep while the intermediate-acting compounds are more effective in maintaining sleep, controlled studies have failed to demonstrate these differential effects. Therefore, as sleep medications, the barbiturates are of limited value beyond short-term use.
Barbiturates have little analgesic action at subanesthetic doses. Rather, in subanesthetic doses these drugs may increase the reaction to painful stimuli. All barbiturates exhibit anticonvulsant activity in anesthetic doses.
Barbiturates are respiratory depressants by virtue of their direct effect on the medullary respiratory center. They diminish and, in high doses, may abolish the sensitivity of the respiratory center to its normal stimulus, carbon dioxide. The degree of respiratory depression is dependent upon dose. With hypnotic doses, respiratory depression produced by barbiturates is similar to that which occurs during physiologic sleep with slight decrease in blood pressure and heart rate.
Ordinary hypnotic doses of barbiturates have no significant effect on the cardiovascular system. The barbiturates tend to decrease the tonus of the gastrointestinal musculature. They have no direct injurious effect on the normal kidney. Severe oliguria or anuria may occur in acute barbiturate poisoning, largely as a result of the marked hypotension.
Hypnotic doses tend to reduce slightly the metabolic rate in man. Body temperature is reduced slightly, owing to lessened activity and to depression of the central temperature-regulatory mechanisms.
While anesthetic doses of all barbiturates exert an anticonvulsant effect, phenobarbital has a selective anticonvulsant activity independent of the degree of sedation produced. Phenobarbital limits the spread of seizures and raises the seizure threshold in grand mal (generalized tonic-clonic) epilepsy.
Studies in laboratory animals have shown that barbiturates cause reduction in the tone and contractility of the uterus, ureters and urinary bladder. However, concentrations of the drugs required to produce this effect in humans are not reached with sedative-hypnoticdoses.
Barbiturates do not impair normal hepatic function, but have been shown to induce liver microsomal enzymes, thus increasing and/or altering the metabolism of barbiturates and other drugs. (See PRECAUTIONS, Drug Interactions.)
Following IV administration, the onset of action is 5 minutes for phenobarbital sodium. For IM administration, the onset of action is slightly slower. Maximal CNS depression may not occur until 15 minutes or more after IV administration.
Duration of action, which is related to the rate at which the barbiturates are redistributed throughout the body, varies among persons and in the same person from time to time.
No studies have demonstrated that the different routes of administration are equivalent with respect to bioavailability.
Barbiturates are weak acids that are absorbed and rapidly distributed to all tissues and fluids with high concentrations in the brain, liver and kidneys. Lipid solubility of the barbiturates is the dominant factor in their distribution within the body. The more lipid soluble the barbiturate, the more rapidly it penetrates all tissues of the body. Barbiturates are bound to plasma and tissue proteins to a varying degree with the degree of binding increasing directly as a function of lipid solubility.
Phenobarbital has the lowest lipid solubility, lowest plasma binding, lowest brain protein binding, the longest delay in onset of activity and the longest duration of action. Its diffusion across the blood-brain barrier and its distribution into other tissues occurs more slowly than with other short-acting barbiturates. Fifteen minutes or more may be required for maximal central depression following intravenous administration of phenobarbital. However, with time, phenobarbital distributes into all tissues and fluids. Barbiturates are known to cross the placenta. Phenobarbital is 20-45% protein bound. In adults, the plasma half-life of phenobarbital is 53 to 118 hours (mean 79 hours) and in children/newborns, the plasma half-life is 60 to 180 hours (mean 110 hours).
Barbiturates are metabolized primarily by the hepatic microsomal enzyme system, and the metabolic products are excreted in the urine and, less commonly, in the feces. Approximately 25 to 50 percent of a dose of phenobarbital is eliminated unchanged in the urine, whereas the amount of other barbiturates excreted unchanged in the urine is negligible. Urinary pH and rate of urine flow affect the renal circulation of unchanged phenobarbital, a greater quantity being eliminated in alkaline urine and at increased flow rates. The excretion of unmetabolized barbiturate is one feature that distinguishes the long-acting category from those belonging to other categories which are almost entirely metabolized. The inactive metabolites of the barbiturates are excreted as conjugates of glucuronic acid.
-
INDICATIONS AND USAGE
Parenteral
- a. Sedative. Sedation is obtainable within an hour, and in adequate dosage, the duration of action is more than six hours. Included in the more common conditions in which the sedative action of this class of drugs is desired are anxiety-tension states, hyperthyroidism, essential hypertension, nausea and vomiting of functional origin, motion sickness, acute labyrinthitis, pylorospasm in infants, chorea and cardiac failure. Phenobarbital is also a useful adjunct in treatment of hemorrhage from the respiratory or gastrointestinal tract. Phenobarbital controls anxiety, decreases muscular activity and lessens nervous excitability in hyperthyroid patients. However, thyrotoxic individuals occasionally react poorly to barbiturates.
- b. Hypnotic, for the short-term treatment of insomnia, since it appears to lose its effectiveness for sleep induction and sleep maintenance after 2 weeks (see CLINICAL PHARMACOLOGY).
- c. Preanesthetic.
- d. Long-term anticonvulsant, (phenobarbital, mephobarbital and metharbital) for the treatment of generalized tonic-clonic and cortical focal seizures. And, in the emergency control of certain acute convulsive episodes, e.g., those associated with status epilepticus, cholera, eclampsia, cerebral hemorrhage, meningitis, tetanus, and toxic reactions to strychnine or local anesthetics. Phenobarbital sodium may be administered intramuscularly or intravenously as an anticonvulsant for emergency use. When administered intravenously, it may require 15 or more minutes before reaching peak concentrations in the brain. Therefore, injecting phenobarbital sodium until the convulsions stop may cause the brain level to exceed that required to control the convulsions and lead to severe barbiturate-induced depression.
- e. Phenobarbital is indicated in pediatric patients as an anticonvulsant and as a sedative, including its preoperative and postoperative use.
-
CONTRAINDICATIONS
Barbiturates are contraindicated in patients with known barbiturate sensitivity. Barbiturates are also contraindicated in patients with a history of manifest or latent porphyria, marked impairment of liver functions or with severe respiratory distress where dyspnea or obstruction is evident. Large doses are contraindicated in nephritic subjects.
Barbiturates should not be administered to persons with known previous addiction to the sedative-hypnotic group since ordinary doses may be ineffectual and may contribute to further addiction.
Intraarterial administration is contraindicated. Its consequences vary from transient pain to gangrene. Subcutaneous administration produces tissue irritation, ranging from tenderness and redness to necrosis and is not recommended. (See DOSAGE AND ADMINISTRATION, Treatment of Adverse Effects Due to Inadvertent Error in Administration.)
Phenobarbital Sodium Injection contains the preservative benzyl alcohol and is not recommended for use in neonates. There have been reports of fatal 'gasping syndrome' in neonates (children less than one month of age) following the administration of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.
Habit Forming
Barbiturates may be habit forming. Tolerance and psychological and physical dependence may occur with continued use (see DRUG ABUSE AND DEPENDENCE and CLINICAL PHARMACOLOGY). Patients who are psychologically dependent on barbiturates may increase the dosage or decrease the dosage interval without consulting a physician and may subsequently develop a physical dependence on barbiturates. To minimize the possibility of overdosage or the development of dependence, the prescribing and dispensing of sedative-hypnotic barbiturates should be limited to the amount required for the interval until the next appointment. Abrupt cessation after prolonged use in the dependent person may result in withdrawal symptoms, including delirium, convulsions and possibly death. Barbiturates should be withdrawn gradually from any patient known to be taking excessive dosage over long periods of time (see DRUG ABUSE AND DEPENDENCE).
Dermatologic Reactions
Exfoliative dermatitis and Stevens-Johnson syndrome, possibly fatal, are rare hypersensitivity reactions to phenobarbital. Physicians should be alert to signs which may precede the onset of barbiturate- induced cutaneous lesions, and the drug should be discontinued whenever dermatological reactions occur.
Intravenous Administration
Too rapid administration may cause severe respiratory depression, apnea, laryngospasm, hypertension or vasodilation with fall in blood pressure.
When administered intravenously, it may require 15 or more minutes before reaching peak concentrations in the brain. Therefore, injecting phenobarbital sodium until the convulsions stop may cause brain levels to exceed that required to control the convulsions and lead to severe barbiturate-induced depression.
Acute or Chronic Pain
Caution should be exercised when barbiturates are administered to patients with acute or chronic pain, because paradoxical excitement could be induced or important symptoms could be masked. However, the use of barbiturates as sedatives in the postoperative surgical period and as adjuncts to cancer chemotherapy is well established.
Use in Pregnancy
Barbiturates can cause fetal harm when administered to a pregnant woman. Retrospective, case-controlled studies have suggested a connection between the maternal consumption of barbiturates and a higher than expected incidence of fetal abnormalities. Phenobarbital may cause major fetal malformations.
Following oral or parenteral administration, barbiturates readily cross the placental barrier and are distributed throughout fetal tissues with highest concentrations found in the placenta, fetal liver and brain. Fetal blood levels approach maternal blood levels following parenteral administration.
Withdrawal symptoms occur in infants born to mothers who receive barbiturates throughout the last trimester of pregnancy (see DRUG ABUSE AND DEPENDENCE).
Phenobarbital should be used during pregnancy only when clearly indicated. If phenobarbital is used during pregnancy or if the patient becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus.
-
PRECAUTIONS
General
Untoward reactions may occur in the presence of fever, hyperthyroidism, diabetes mellitus and severe anemia. In cases of great debility, severely impaired liver function, pulmonary or cardiac disease, status asthmaticus, shock or uremia, phenobarbital sodium should be used with extreme caution. Intramuscular injection should be confined to a total volume of 5 mL and made in a large muscle in order to avoid possible tissue irritation.
Barbiturates may be habit forming. Tolerance and psychological and physical dependence may occur with continued use (see DRUG ABUSE AND DEPENDENCE). Barbiturates should be administered with caution, if at all, to patients who are mentally depressed, have suicidal tendencies or a history of drug abuse.
Elderly or debilitated patients may react to barbiturates with marked excitement, depression and confusion. In some persons, barbiturates repeatedly produce excitement rather than depression.
In patients with hepatic damage, barbiturates should be administered with caution and initially in reduced doses. Barbiturates should not be administered to patients showing the premonitory signs of hepatic coma.
Parenteral solutions of barbiturates are highly alkaline. Therefore, extreme care should be taken to avoid perivascular extravasation or intraarterial injection. Extravascular injection may cause local tissue damage with subsequent necrosis; consequences of intraarterial injection may vary from transient pain to gangrene of the limb. Any complaint of pain in the limb warrants stopping the injection.
Information for the Patient
Practitioners should give the following information and instructions to patients receiving barbiturates:
- The use of barbiturates carries with it an associated risk of psychological and/or physical dependence. The patient should be warned against increasing the dose of the drug without consulting a physician.
- Barbiturates may impair mental and/or physical abilities required for the performance of potentially hazardous tasks (e.g., driving, operating machinery, etc.).
- Alcohol should not be consumed while taking barbiturates. Concurrent use of the barbiturates with other CNS depressants (e.g., alcohol, narcotics, tranquilizers and antihistamines) may result in additional CNS depressant effects.
- If phenobarbital is used during pregnancy, the patient should be apprised of the potential hazard to the fetus (see CONTRAINDICATIONS).
Drug Interactions
Most reports of clinically significant drug interactions occurring with the barbiturates have involved phenobarbital. However, the application of these data to other barbiturates appears valid and warrants serial blood level determinations of the relevant drugs when there are multiple therapies.
Anticoagulants
Phenobarbital lowers the plasma levels of dicumarol (bishydroxycoumarin) and causes a decrease in anticoagulant activity as measured by the prothrombin time. Barbiturates can induce hepatic microsomal enzymes resulting in increased metabolism and decreased anticoagulant response of oral anticoagulants (e.g., warfarin, acenocoumarol, dicumarol and phenprocoumon). Patients stabilized on anticoagulant therapy may require dosage adjustments if barbiturates are added to or withdrawn from their dosage regimen.
Corticosteroids
Barbiturates appear to enhance the metabolism of exogenous corticosteroids probably through the induction of hepatic microsomal enzymes. Patients stabilized on corticosteroid therapy may require dosage adjustments if barbiturates are added to or withdrawn from thier dosage regimen.
Griseofulvin
Phenobarbital appears to interfere with the absorption of orally administered griseofulvin, thus decreasing its blood level. The effect of the resultant decreased blood levels of griseofulvin on therapeutic response has not been established. However, it would be preferable to avoid concomitant administration of these drugs.
Doxycycline
Phenobarbital has been shown to shorten the half-life of doxycycline for as long as 2 weeks after barbiturate therapy is discontinued. This mechanism is probably through the induction of hepatic microsomal enzymes that metabolize the antibiotic. If phenobarbital and doxycycline are administered concurrently, the clinical response to doxycycline should be monitored closely.
Phenytoin, Sodium Valproate, Valproic Acid
The effect of barbiturates on the metabolism of phenytoin appears to be variable. Some investigators report an accelerating effect, while others report no effect. Because the effect of barbiturates on the metabolism of phenytoin is not predictable, phenytoin and barbiturate blood levels should be monitored more frequently if these drugs are given concurrently. Sodium valproate and valproic acid appear to decrease barbiturate metabolism; therefore, barbiturate blood levels should be monitored and appropriate dosage adjustments made as indicated.
Estradiol, Estrone, Progesterone and Other Steroidal Hormones
Pretreatment with or concurrent administration of phenobarbital may decrease the effect of estradiol by increasing its metabolism. There have been reports of patients treated with antiepileptic drugs (e.g., phenobarbital) who became pregnant while taking oral contraceptives. An alternate contraceptive method might be suggested to women taking phenobarbital.
Drug/Laboratory Test Interactions
Prolonged therapy with barbiturates should be accompanied by periodic laboratory evaluation of organ systems, including hematopoietic, renal and hepatic systems (see PRECAUTIONS, General and ADVERSE REACTIONS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal Data
Phenobarbital sodium is carcinogenic in mice and rats after lifetime administration. In mice, it produced benign and malignant liver-cell tumors. In rats, benign liver-cell tumors were observed very late in life.
Human Data
In an epidemiological study of 9,136 patients who were treated on an anticonvulsant protocol which included phenobarbital, results indicated a higher than normal incidence of hepatic carcinoma.
Previously, some of these patients were treated with thorotrast, a drug which is known to produce hepatic carcinomas. Thus, this study did not provide sufficient evidence that phenobarbital is carcinogenic in humans. A retrospective study of 84 children with brain tumors matched to 73 normal controls and 78 cancer controls (malignant disease other than brain tumors) suggested an association between exposure to barbiturates prenatally and an increased incidence of brain tumors.
Pregnancy
Teratogenic Effects-Pregnancy Category D
Phenobarbital may cause major fetal malformations. (See CONTRAINDICATIONS, Use in Pregnancy.)
Nonteratogenic Effects
Reports of infants suffering from long-term barbiturate exposure in utero included the acute withdrawal syndrome of seizures and hyperirritability from birth to a delayed onset of up to 14 days. (See DRUG ABUSE AND DEPENDENCE.)
Labor and Delivery
Hypnotic doses of barbiturates do not appear to significantly impair uterine activity during labor. Full anesthetic doses of barbiturates decrease the force and frequency of uterine contractions. Administration of sedative-hypnotic barbiturates to the mother during labor may result in respiratory depression in the newborn. Premature infants are particularly susceptible to the depressant effects of barbiturates. If barbiturates are used during labor and delivery, resuscitation equipment should be available.
Barbiturates are additive with other central nervous system depressants administered during labor and delivery. Data are currently not available to evaluate the effect of barbiturates when forceps delivery or other intervention is necessary or to determine the long-term effects of obstetrically administered barbiturates on the later growth, development and functional maturation of the child.
While barbiturates are usually reported to have a minimal effect on neonatal behavior, a delayed interest in breastfeeding and a depressed response to auditory and visual stimuli have been noted.
-
ADVERSE REACTIONS
The following adverse reactions and their incidence were compiled from surveillance of thousands of hospitalized patients. Because such patients may be less aware of certain of the milder adverse effects of barbiturates, the incidence of these reactions may be somewhat higher in fully ambulatory patients.
Nervous System
Somnolence, agitation, confusion, hyperkinesia, ataxia, CNS depression, nightmares, nervousness, psychiatric disturbance, hallucinations, insomnia, anxiety, dizziness, thinking abnormality
-
DRUG ABUSE AND DEPENDENCE
Phenobarbital Sodium Injection is a Schedule IV controlled substance.
Barbiturates may be habit forming. Tolerance and psychological dependence and physical dependence may occur especially following prolonged use of high doses of barbiturates. As tolerance to barbiturates develops, the amount needed to maintain the same level of intoxication increases; tolerance to a fatal dosage, however, does not increase more than two-fold. As this occurs, the margin between an intoxicating dosage and fatal dosage becomes smaller. Symptoms of acute intoxication with barbiturates include unsteady gait, slurred speech and sustained nystagmus. Mental signs of chronic intoxication include confusion, poor judgment, irritability, insomnia and somatic complaints. Symptoms of barbiturate dependence are similar to those of chronic alcoholism. If an individual appears to be intoxicated with alcohol to a degree that is radically disproportionate to the amount of alcohol in his or her blood, the use of barbiturates should be suspected. The lethal dose of a barbiturate is far less if alcohol is also ingested.
The symptoms of barbiturate withdrawal can be severe and may cause death. Minor withdrawal symptoms may appear 8 to 12 hours after the last dose of a barbiturate. These symptoms usually appear in the following order: anxiety, muscle twitching, tremor of hands and fingers, progressive weakness, dizziness, distortion in visual perception, nausea, vomiting, insomnia and orthostatic hypotension. Major withdrawal symptoms (convulsions and delirium) may occur within 16 hours and last up to 5 days after abrupt cessation of these drugs. Intensity of withdrawal symptoms gradually declines over a period of approximately 15 days. Individuals susceptible to barbiturate abuse and dependence include alcoholics and opiate abusers, as well as other sedative-hypnotic and amphetamine abusers.
Drug dependence to barbiturates arises from repeated administration of a barbiturate or agent with barbiturate-like effect on a continuous basis, generally in amounts exceeding therapeutic dose levels. The characteristics of drug dependence to barbiturates include: (a) a strong desire or need to continue taking the drug, (b) a tendency to increase the dose, (c) a psychic dependence on the effects of the drug related to subjective and individual appreciation of those effects and (d) a physical dependence on the effects of the drug requiring its presence for maintenance of homeostasis and resulting in a definite, characteristic and self-limited abstinence syndrome when the drug is withdrawn.
Individuals subject to barbiturate abuse and dependence include alcoholics and opiate abusers as well as other sedative-hypnotics and amphetamine abusers.
Treatment of barbiturate dependence consists of cautious and gradual withdrawal of the drug. Barbiturate-dependent patients can be withdrawn by using a number of different withdrawal regimens. In all cases, withdrawal takes an extended period of time. One method involves substituting a 30 mg dose of phenobarbital for each 100 to 200 mg dose of barbiturate that the patient has been taking. The total daily amount of phenobarbital is then administered in 3 to 4 divided doses, not to exceed 600 mg daily. Should signs of withdrawal occur on the first day of trearment, a loading dose of 100 to 200 mg of phenobarbital may be administered IM in addition to the oral dose. After stabilization on phenobarbital, the total daily dose is decreased by 30 mg a day as long as withdrawal is proceeding smoothly. If withdrawal symptoms appear, dosage is maintained at that level or increased slightly until symptoms disappear. A modification of this regimen involves initiating treatment at the patient's regular dosage level and decreasing the daily dosage by 10 percent if tolerated by the patient. The symptoms of withdrawal can be severe and may cause death. Minor withdrawal symptoms (e.g., anxiety, muscle twitching, tremors, nausea, etc.) may appear 8-12 hours after the last dose of a barbiturate. Major withdrawal symptoms (convulsions and delirium) may occur within 16 hours and last up to five days after abrupt cessation of the barbiturate. The intensity of withdrawal symptoms gradually declines over a period of two weeks.
Infants physically dependent on barbiturates may be given phenobarbital 3 to 10 mg/kg/day. After withdrawal symptoms (hyperactivity, disturbed sleep, tremors, hyperreflexia) are relieved, the dosage of phenobarbital should be gradually decreased and completely withdrawn over a 2-week period.
-
OVERDOSAGE
The toxic dose of barbiturates varies considerably. Barbiturate intoxication may be confused with alcoholism, bromide intoxication and various neurological disorders.
For sedation, therapeutic blood levels of phenobarbital range from 5-40 μg/mL; the lethal blood level is greater than 80 μg/mL and usually ranges from 100-200 μg/mL.
Acute overdosage with barbiturates is manifested by CNS and respiratory depression which may progress to Cheyne-Stokes respiration, areflexia, constriction of the pupils to a slight degree (though in severe poisoning, they may show paralytic dilation), oliguria, tachycardia, hypotension, lowered body temperature and coma. Typical shock syndrome (apnea, circulatory collapse, respiratory arrest and death) may occur.
In extreme overdose, all electrical activity in the brain may cease, in which case a "flat" EEG normally equated with clinical death cannot be accepted. This effect is fully reversible unless hypoxic damage occurs. Consideration should be given to the possibility of barbiturate intoxication even in situations that appear to involve trauma.
Complications such as pneumonia, pulmonary edema, cardiac arrhythmias, congestive heart failure and renal failure may occur. Uremia may increase CNS sensitivity to barbiturates if renal function is impaired. Differential diagnosis should include hypoglycemia, head trauma, cerebrovascular accidents, convulsive states and diabetic coma. To obtain up-to-date information about the treatment of overdosage, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdose, interaction among drugs and the unusual drug kinetics in your patient.
Treatment of overdosage is mainly supportive and consists of the following:
- Maintenance of an adequate airway, with assisted respiration and oxygen administration as necessary.
- Monitoring of vital signs and fluid balance.
- Fluid therapy and other standard treatment for shock, if needed.
- If renal function is normal, forced diuresis may aid in the elimination of the barbiturate. Alkalinization of the urine increases renal excretion of phenobarbital.
- Although not recommended as a routine procedure, hemodialysis may be used in severe barbiturate intoxication or if the patient is anuric or in shock. Hemoperfusion through an anion-exchange resin or activated charcoal has been successful. Peritoneal dialysis is significantly less effective in removing barbiturates.
- Patient should be rolled from side to side every 30 minutes.
- Antibiotics should be given if pneumonia is suspected.
- Appropriate nursing care to prevent hypostatic pneumonia, decubiti, aspiration and other complications of patients with altered states of consciousness.
- The use of analeptic agents is not recommended.
-
DOSAGE AND ADMINISTRATION
Dosages of barbiturates must be individualized with full knowledge of their particular characteristics and recommended rates of administration. Factors to consider are the patient's age, weight and condition.
Suggested doses of phenobarbital sodium for specific indications follow:
Pediatric Dosage
Recommended by the American Academy of Pediatrics (intended as a guide)
Preoperative Sedation: 1 to 3 mg/kg IM or IV
Anticonvulsant: 4 to 6 mg/kg/day for 7 to 10 days to blood level of 10 to 15 mcg/mL or 10 to 15 mg/kg/day IM or IV
Status Epilepticus: 15 to 20 mg/kg over 10 to 15 minutes IV
Adult Dosage
(intended as a guide)
Daytime Sedation: 30 to 120 mg daily in 2 to 3 divided doses IM or IV
Bedtime Hypnosis: 100 to 320 mg IM or IV
Preoperative Sedation: IM only — 100 to 200 mg 60 to 90 minutes before surgery
Acute Convulsions: 20 to 320 mg IM or IV, repeated in 6 hours as necessary
Parenteral routes should be used only when oral administration is impossible or impractical.
Intramuscular injection of the sodium salts of barbiturates should be made deeply into a large muscle and a volume of 5 mL should not be exceeded at any one site because of possible tissue irritation. Injection into or near peripheral nerves may result in permanent neurological deficit. After intramuscular injection of a hypnotic dose, the patient's vital signs should bemonitored.
Subcutaneous administration is not recommended (see CONTRAINDICATIONS).
Intravenous Administration
Intravenous injection is restricted to conditions in which other routes are not feasible, either because the patient is unconscious (as in cerebral hemorrhage, eclampsia or status epilepticus), or because the patient resists (as in delirium) or because prompt action is imperative. Slow IV injection is essential, and patients should be carefully observed during administration. This requires that blood pressure, respiration and cardiac function be maintained, vital signs be recorded and equipment for resuscitation and artificial ventilation be available. The rate of intravenous injection for adults should not exceed 60 mg/min for phenobarbital sodium.
When given intravenously, do not use small veins, such as those on the dorsum of the hand or wrist. Preference should be given to a larger vein to minimize the risk of irritation with the possibility of resultant thrombosis. Avoid administration into varicose veins because circulation there is retarded. Inadvertent injection into or adjacent to an artery has resulted in gangrene requiring amputation of an extremity or a portion thereof. Careful technique, including aspiration, is necessary to avoid inadvertent intraarterial injection. (See below.)
Treatment of Adverse Effects Due to Inadvertent Error in Administration
Extravasation into subcutaneous tissues causes tissue irritation. This may vary from slight tenderness and redness to necrosis. Recommended treatment includes the application of moist heat and the injection of 0.5% procaine solution into the affected area.
Intraarterial injection of any barbiturate must be avoided. The accidental intraarterial injection of a small amount of the solution may cause spasm and severe pain along the course of the artery. The injection should be terminated if the patient complains of pain or if other indications of accidental intraarterial injection occur, such as a white hand with cyanosed skin or patches of discolored skin and delayed onset of hypnosis.
The consequences of intraarterial injection of phenobarbital can vary from transient pain to gangrene. It is not possible to formulate strict rules for management of such accidents. The following procedures have been suggested: 1) release of the tourniquet or restrictive garments to permit dilution of injected drug, 2) relief of arterial spasm by injecting 10 mL of a 1% procaine solution into the artery and, if considered necessary, brachial plexus block, 3) prevention of thrombosis by early anticoagulant therapy and 4) supportive treatment.
Anticonvulsant Use
A therapeutic anticonvulsant level of phenobarbital in the serum is 10 to 25 μg/mL. To achieve the blood levels considered therapeutic in children, higher per-kilogram dosages are generally necessary for phenobarbital and most other anticonvulsants. In children and infants, phenobarbital at loading doses of 15 to 20 mg/kg produces blood levels of about 20 μg/mL shortly after administration.
In status epilepticus, it is imperative to achieve therapeutic blood levels of a barbiturate (or other anticonvulsants) as rapidly as possible. When administered intravenously, phenobarbital sodium may require 15 minutes or more to attain peak concentrations in the brain. If phenobarbital sodium is injected continuously until the convulsions stop, the brain concentration will continue to rise and can eventually exceed that required to control the seizures. Because a barbiturate-induced depression may occur along with a postictal depression once the seizures are controlled, it is important, therefore, to use the minimal amount required and to wait for the anticonvulsant effect to develop before administering a second dose.
Phenobarbital has been used in the treatment and prophylaxis of febrile seizures. However, it has not been established that prevention of febrile seizures influences the subsequent development of epilepsy.
Special Patient Population
Dosage should be reduced in the elderly or debilitated because these patients may be more sensitive to barbiturates. Dosage should be reduced for patients with impaired renal function or hepatic disease.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Phenobarbital Sodium Injection, USP is available in the following:
65 mg/mL, 1 mL vials packaged in 25s (NDC: 42494-441-25)
130 mg/mL, 1 mL vials packaged in 25s (NDC: 42494-442-25)
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 65 mg/mL Vial Label
NDC: 42494-441-25
CIV
PHENOBARBITAL
Sodium Injection, USP65 mg/mL
Rx onlyFOR IM OR SLOW IV USE
25 x 1 mL Vials
Store at 20°-25°C (68°-77°F)
[See USP Controlled Room Temperature]DO NOT USE IF DISCOLORED OR
CONTAINS A PRECIPITATE.MANUFACTURED FOR: novaplus™
NOVAPLUS IS A REGISTERED TRADEMARK OF VIZIENT, INC.
-
PRINCIPAL DISPLAY PANEL - 130 mg/mL Vial Label
NDC: 42494-442-25
CIV
PHENOBARBITAL
Sodium Injection, USP130 mg/mL
Rx onlyFOR IM OR SLOW IV USE
25 x 1 mL Vials
Store at 20°-25°C (68°-77°F)
[See USP Controlled Room Temperature]DO NOT USE IF DISCOLORED OR
CONTAINS A PRECIPITATE.MANUFACTURED FOR: novaplus™
NOVAPLUS IS A REGISTERED TRADEMARK OF VIZIENT, INC.
-
INGREDIENTS AND APPEARANCE
PHENOBARBITAL SODIUM
phenobarbital sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42494-441 Route of Administration INTRAMUSCULAR DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL SODIUM (UNII: SW9M9BB5K3) (Phenobarbital - UNII:YQE403BP4D) PHENOBARBITAL SODIUM 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Propylene Glycol (UNII: 6DC9Q167V3) Benzyl Alcohol (UNII: LKG8494WBH) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42494-441-25 25 in 1 BOX, UNIT-DOSE 06/02/2023 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/02/2023 PHENOBARBITAL SODIUM
phenobarbital sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42494-442 Route of Administration INTRAMUSCULAR DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL SODIUM (UNII: SW9M9BB5K3) (Phenobarbital - UNII:YQE403BP4D) PHENOBARBITAL SODIUM 130 mg in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Propylene Glycol (UNII: 6DC9Q167V3) Benzyl Alcohol (UNII: LKG8494WBH) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42494-442-25 3 in 1 BOX, UNIT-DOSE 06/02/2023 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/02/2023 Labeler - Cameron Pharmaceuticals (078371442) Establishment Name Address ID/FEI Business Operations Vitae Enim Vitae Scientific, Inc. 080492645 MANUFACTURE(42494-441, 42494-442)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.