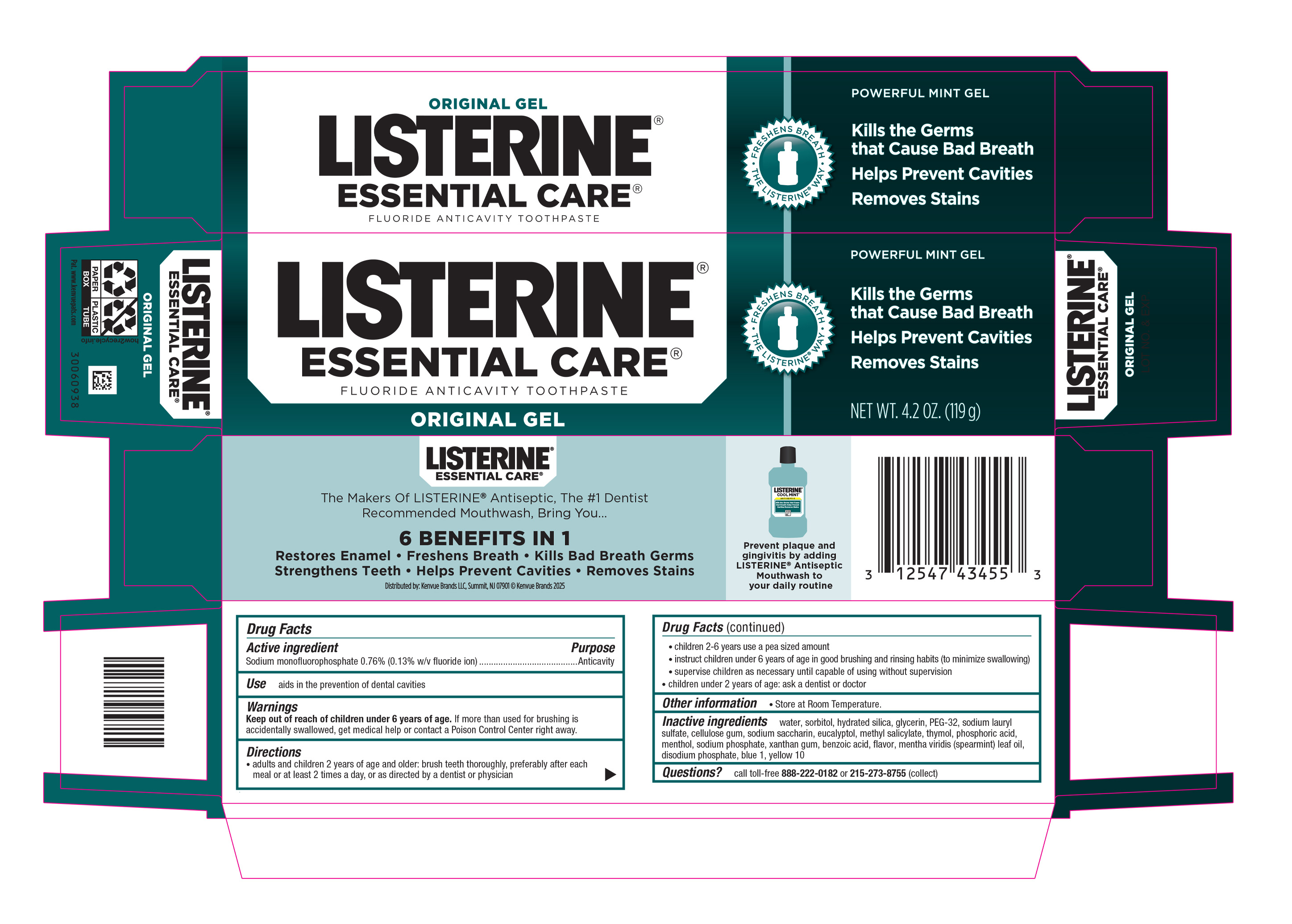
LISTERINE ESSENTIAL CARE- sodium monofluorophosphate gel, dentifrice
Listerine Essential Care by
Drug Labeling and Warnings
Listerine Essential Care by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least 2 times a day, or as directed by a dentist or physician.
- children 2-6 years use a pea sized amount
- instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision.
- children under 2 years of age: ask a dentist or doctor
- adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least 2 times a day, or as directed by a dentist or physician.
- Other information
-
Inactive ingredients
water, sorbitol, hydrated silica, glycerin, PEG-32, sodium lauryl sulfate, cellulose gum, sodium saccharin, eucalyptol, methyl salicylate, thymol, phosphoric acid, menthol, sodium phosphate, xanthan gum, benzoic acid, flavor, mentha viridis (spearmint) leaf oil, disodium phosphate, blue 1, yellow 10
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 119 g Tube Carton
-
INGREDIENTS AND APPEARANCE
LISTERINE ESSENTIAL CARE
sodium monofluorophosphate gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69968-0701 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.3 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) HYDRATED SILICA (UNII: Y6O7T4G8P9) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 1600 (UNII: 1212Z7S33A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SACCHARIN SODIUM (UNII: SB8ZUX40TY) EUCALYPTOL (UNII: RV6J6604TK) METHYL SALICYLATE (UNII: LAV5U5022Y) THYMOL (UNII: 3J50XA376E) PHOSPHORIC ACID (UNII: E4GA8884NN) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) SODIUM PHOSPHATE (UNII: SE337SVY37) XANTHAN GUM (UNII: TTV12P4NEE) BENZOIC ACID (UNII: 8SKN0B0MIM) SPEARMINT OIL (UNII: C3M81465G5) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69968-0701-4 1 in 1 CARTON 03/03/2021 1 119 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 03/03/2021 Labeler - Kenvue Brands LLC (118772437)
Trademark Results [Listerine Essential Care]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LISTERINE ESSENTIAL CARE 85860070 not registered Dead/Abandoned |
Johnson & johnson 2013-02-26 |
 LISTERINE ESSENTIAL CARE 77591101 3606056 Live/Registered |
Johnson & Johnson 2008-10-13 |
 LISTERINE ESSENTIAL CARE 75558423 2488147 Dead/Cancelled |
Warner-Lambert Company 1998-09-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.