Description of Sildenafil 5 mg and 100 mg Tablets
11. DESCRIPTION Sildenafil Tablets, USP phosphodiesterase-5 (PDE-5) inhibitor, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type-5 (PDE-5). Sildenafil is also marketed as VIAGRA for erectile dysfunction. Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
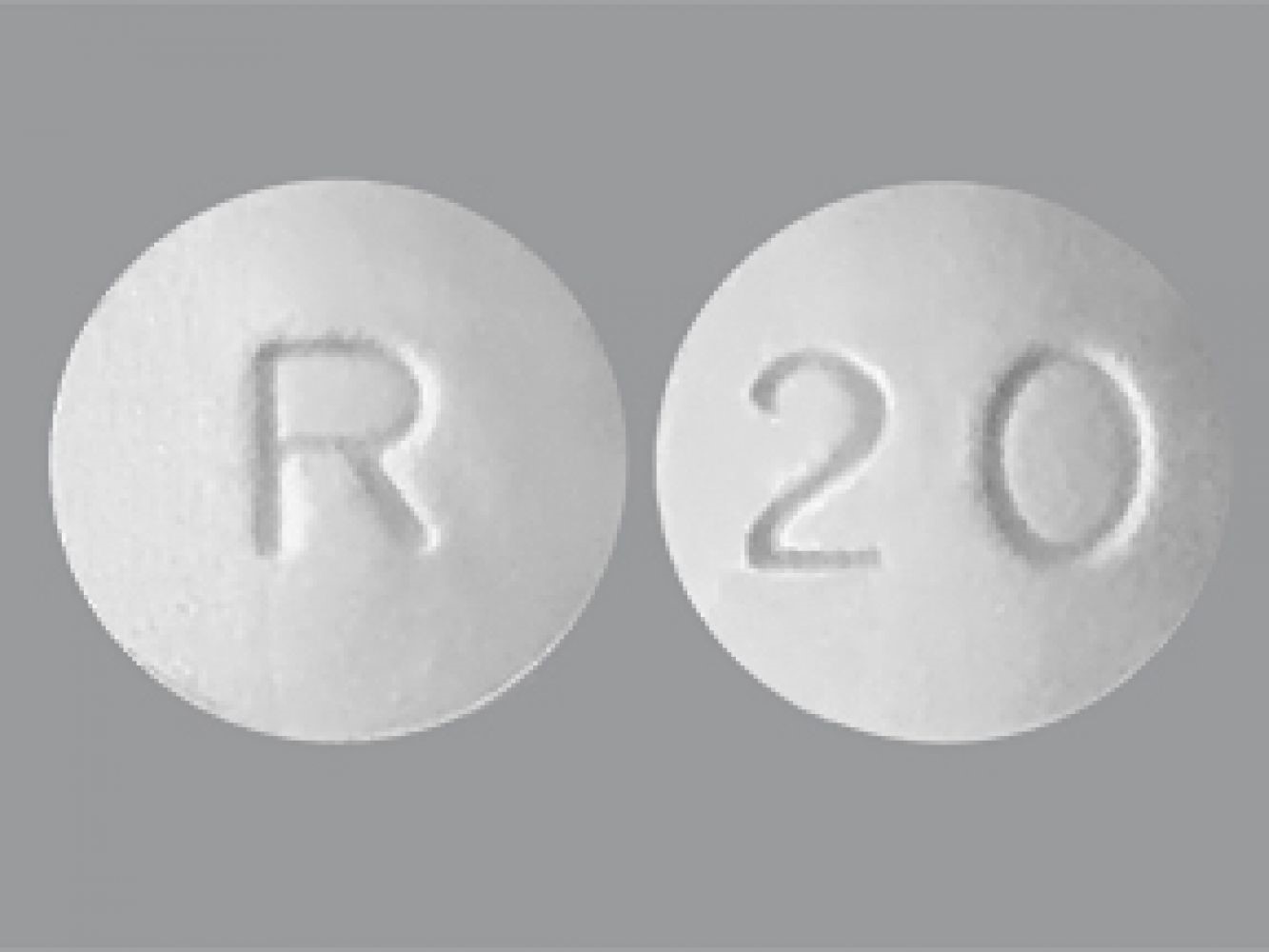
Sildenafil citrate is a white or almost white, slightly hygroscopic crystalline powder. Slightly soluble in water & methanol, practically insoluble in hexane and a molecular weight of 666.7. Sildenafil tablets: Sildenafil tablets is white round, biconvex film coated tablets, debossed with R on one side and 20 on the other, containing sildenafil citrate equivalent to 20 mg of sildenafil. In addition to the active ingredient, sildenafil citrate , each tablet contains the following inactive ingredients: Crospovidone, Hypromellose, Hydrophobic colloidal silica, Lactose monohydrate, Magnesium stearate, Microcrystalline cellulose, Titanium dioxide, and Triacetin.
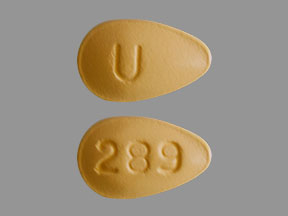
Description of Tadalafil 5 mg and 20 mg Tablets
Tadalafil USP is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C H N O representing a molecular weight of 389.41. The structural formula is:
The chemical designation is pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione,6-(1,3-benzodioxol-5yl)2,3,6,7,12,12a-hexahydro-2-methyl-,(6R,12aR)-. It is a crystalline solid that is freely soluble in dimethyl sulfoxide, slightly soluble in methylene chloride and practically insoluble in water Tadalafil Tablets USP are available as oval / almond shaped film coated tablets for oral administration. Each tablet contains 2.5, 5, 10, or 20 mg of tadalafil and the following inactive ingredients : Lactose monohydrate, Microcrystalline Cellulose, Sodium Lauryl Sulphate, Croscarmellose Sodium, Hydroxypropyl Cellulose, Magnesium Stearate, Hypromellose, Titanium Dioxide, Triacetin, Talc and Yellow iron oxide. In addition, Tadalafil Tablets, 2.5 mg and 10 mg contains Red iron oxide
Sildenafil 20 mg and 100 mg oackage label.


Tadalafil 5 mg and 20 mg Tablet Label