SUN SPR SPF 50- avobenzone,octinoxate,octocrylene,oxybenzone,titanium dioxide spray
SUN SPR by
Drug Labeling and Warnings
SUN SPR by is a Otc medication manufactured, distributed, or labeled by AGEFARM SRL, COSMESIT SRL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
1apply liberally 15 minutes before the sun exposure
reapplyat least every 2 hours, immediately after towel drying
use a water-resistant sunscreen if swimming or sweating
children under 6 months Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including
limit the time in the sun, especially from 10 a.m. - 2p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
- 1 SUNSCREEN 5033 BODY SPRAY SPF 50.jpg
-
INACTIVE INGREDIENTS
alcohol, BHT, bis-ethylhexyloxyphenol methoxphenyl triazine, butyrospermum parkii butter extract, caprylic - capric triglyceride, carthamus tinctorius seed oil, cellulose gum, citronellol, coumarin, cyclomethicone, c12-15 alkyl benzoate, decyl glucose, diisopropyl sebacate, dimethicone, glycerin, hexyl cinnamal, coumarin, hydrolyzed soy protein, hydrolyzed whaet protein, lecithin, limnanthes alba seed oil, limonene, linalool, methylene bis-benzotriazoly tetramethybutyphenol (nano), methylisothizolinone, menthyl lactate, microcrystalline cellulose, olea europapea oil unsonifiables, parfum, phenoxyhetanol, potassium cetyl phosphate, propylene gycol, silica, tocopheryl acetate, tripeptide-1, vp-eicosene copolymer, water, xanthan gum
- STORAGE AND HANDLING
- PRINCIPAL LABEL
-
INGREDIENTS AND APPEARANCE
SUN SPR SPF 50
avobenzone,octinoxate,octocrylene,oxybenzone,titanium dioxide sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73310-5033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1 mg in 5 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1 mg in 5 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1 mg in 5 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 mg in 5 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 mg in 5 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1 mg in 5 mL Product Characteristics Color white (CREAM) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73310-5033-3 150 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/28/2019 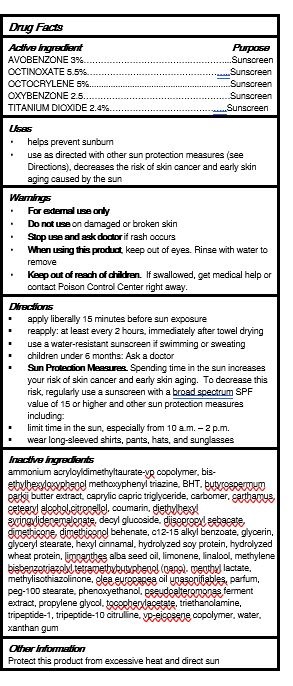
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/28/2019 Labeler - AGEFARM SRL (439202467) Establishment Name Address ID/FEI Business Operations COSMESIT SRL 339071951 manufacture(73310-5033)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 SUNSCREEN 5033 BODY SPF50.jpg
SUNSCREEN 5033 BODY SPF50.jpg