MESNA tablet, film coated
MESNA by
Drug Labeling and Warnings
MESNA by is a Prescription medication manufactured, distributed, or labeled by Ingenus Pharmaceuticals, LLC, RiconPharma LLC, Ingenus Pharmaceuticals NJ, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESNA TABLETS safely and effectively. See full prescribing information for MESNA TABLETS.
MESNA tablets, for oral use
Initial U.S. Approval: 1988RECENT MAJOR CHANGES
Warnings and Precautions, Benzyl Alcohol Toxicity (5.3) 12/2018
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Mesna may be given as a fractionated dosing schedule of a single bolus injection followed by two oral administrations of mesna tablets as outlined below. The dosing schedule should be repeated on each day that ifosfamide is administered. When the dosage of ifosfamide is adjusted, the ratio of mesna to ifosfamide should be maintained. (2)
Intravenous and Oral Dosing Schedule:
0 Hours
2 Hours
6 Hours
Ifosfamide
1.2 g/m2
--
--
MESNEX Injection
240 mg/m2
--
--
Mesna Tablets
--
480 mg/m2
480 mg/m2
Maintain sufficient urinary output, as required for ifosfamide treatment, and monitor urine for the presence of hematuria. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 400 mg with functional score (3)
CONTRAINDICATIONS
Known hypersensitivity to mesna or to any of the excipients in mesna tablets and MESNEX injection, including benzyl alcohol. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Anaphylactic reactions have been reported. Less severe hypersensitivity reactions may also occur. Monitor patients. If a reaction occurs, discontinue mesna and provide supportive care. (5.1)
- Dermatologic toxicity: Skin rash with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, and toxic epidermal necrolysis have occurred. Skin rash, urticaria, and angioedema have also been seen. Monitor patients. If a reaction occurs, discontinue mesna and provide supportive care. (5.2)
- Benzyl alcohol toxicity: Serious and fatal adverse reactions can occur in premature neonates and low-birth weight infants treated with benzyl alcohol-preserved drugs, including MESNEX injection. Avoid use in premature neonates and low-birth weight infants. (5.3)
- Laboratory test alterations: False positive tests for urinary ketones and interference with enzymatic CPK activity tests have been seen. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (> 10%) when mesna is given with ifosfamide are nausea, vomiting, constipation, leukopenia, fatigue, fever, anorexia, thrombocytopenia, anemia, granulocytopenia, diarrhea, asthenia, abdominal pain, headache, alopecia, and somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Mesna in combination with ifosfamide can cause fetal harm. Advise patients of potential risk to a fetus. (8.1)
- Lactation: Do not breastfeed. (8.2)
- Females and Males of Reproductive Potential: Advise patients to use effective contraception. Verify pregnancy status prior to initiation of mesna in combination with ifosfamide. (8.3)
- Pediatric use: In premature neonates and low-birth weight infants, avoid use of benzyl alcohol–containing solutions. (8.4)
- Geriatric use: Dose selection should be cautious. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.2 Intravenous and Oral Dosing
2.3 Monitoring for Hematuria
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Dermatologic Toxicity
5.3 Benzyl Alcohol Toxicity
5.4 Laboratory Test Interferences
5.5 Use in Patients with a History of Adverse Reactions to Thiol Compounds
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
8.7 Use in Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.2 Oral Mesna
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.2 Intravenous and Oral Dosing
Mesna may be given on a fractionated dosing schedule of a single bolus injection followed by two oral administrations of mesna tablets as outlined below.
MESNEX injection is given as intravenous bolus injections in a dosage equal to 20% of the ifosfamide dosage (w/w) at the time of ifosfamide administration. Mesna tablets are given orally in a dosage equal to 40% of the ifosfamide dose 2 and 6 hours after each dose of ifosfamide. The total daily dose of mesna is 100% of the ifosfamide dose. The recommended dosing schedule is outlined in Table 2.
Table 2. Recommended Intravenous and Oral Dosing Schedule - * The dosing schedule should be repeated on each day that ifosfamide is administered. When the dosage of ifosfamide is increased or decreased, the ratio of mesna to ifosfamide should be maintained.
0 Hours
2 Hours
6 Hours
Ifosfamide
1.2 g/m2
–
–
MESNEX Injection*
240 mg/m2
–
–
Mesna Tablets
–
480 mg/m2
480 mg/m2
The efficacy and safety of this ratio of intravenous and oral mesna has not been established as being effective for daily doses of ifosfamide higher than 2 g/m2.
Patients who vomit within two hours of taking oral mesna should repeat the dose or receive intravenous MESNEX.
2.3 Monitoring for Hematuria
Maintain adequate hydration and sufficient urinary output, as required for ifosfamide treatment, and monitor urine for the presence of hematuria. If severe hematuria develops when mesna is given according to the recommended dosage schedule, dosage reductions or discontinuation of ifosfamide therapy may be required.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Mesna is contraindicated in patients known to be hypersensitive to mesna or to any of the excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Mesna may cause systemic hypersensitivity reactions, including anaphylaxis. These reactions may include fever, cardiovascular symptoms (hypotension, tachycardia), acute renal impairment, hypoxia, respiratory distress, urticaria, angioedema, laboratory signs of disseminated intravascular coagulation, hematological abnormalities, increased liver enzymes, nausea, vomiting, arthralgia, and myalgia. These reactions may occur with the first exposure or after several months of exposure. Monitor for signs or symptoms. Discontinue mesna and provide supportive care.
5.2 Dermatologic Toxicity
Drug rash with eosinophilia and systemic symptoms and bullous and ulcerative skin and mucosal reactions, consistent with Stevens-Johnson syndrome or toxic epidermal necrolysis have occurred. Mesna may cause skin and mucosal reactions characterized by urticaria, rash, erythema, pruritus, burning sensation, angioedema, periorbital edema, flushing and stomatitis. These reactions may occur with the first exposure or after several months of exposure. Discontinue mesna and provide supportive care.
5.3 Benzyl Alcohol Toxicity
Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and low-birth weight infants who received benzyl alcohol dosages of 99 to 234 mg/kg/day (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Symptoms associated with “gasping syndrome” and other potential adverse reactions include gradual neurological deterioration, seizures, intracranial hemorrhage, hematological abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Premature neonates and low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which toxicity may occur is not known. MESNEX injection contains 10.4 mg/mL of the preservative benzyl alcohol. Avoid use of MESNEX injection in premature neonates and low-birth weight infants. Mesna tablets do not contain benzyl alcohol [see Use in Specific Populations (8.4)].
5.4 Laboratory Test Interferences
False-Positive Urine Tests for Ketone Bodies
A false positive test for urinary ketones may arise in patients treated with mesna when using nitroprusside sodium-based urine tests (including dipstick tests). The addition of glacial acetic acid can be used to differentiate between a false positive result (cherry-red color that fades) and a true positive result (red-violet color that intensifies).
False-Negative Tests for Enzymatic CPK Activity
Mesna may interfere with enzymatic creatinine phosphokinase (CPK) activity tests that use a thiol compound (e.g., N-acetylcysteine) for CPK reactivation. This may result in a falsely low CPK level.
False-Positive Tests for Ascorbic Acid
Mesna may cause false-positive reactions in Tillman’s reagent-based urine screening tests for ascorbic acid.
5.5 Use in Patients with a History of Adverse Reactions to Thiol Compounds
Mesna is a thiol compound, i.e., a sulfhydryl (SH) group-containing organic compound. Hypersensitivity reactions to mesna and to amifostine, another thiol compound, have been reported. It is not clear whether patients who experienced an adverse reaction to a thiol compound are at increased risk for a hypersensitivity reaction to mesna.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling.
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Dermatological Toxicity [see Warnings and Precautions (5.2)]
- Benzyl Alcohol Toxicity [see Warnings and Precautions (5.3)]
- Laboratory Test Interferences [see Warnings and Precautions (5.4)]
- Use in Patients with a History of Adverse Reactions to Thiol Compounds [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Mesna adverse reaction data are available from four Phase 1 studies in which single oral doses of 600-2400 mg of mesna tablets were administered to a total of 82 healthy volunteers. In two Phase 1 multiple-dose studies where healthy volunteers received mesna tablets alone or intravenous MESNEX followed by repeated doses of mesna tablets, flatulence and rhinitis were reported.
Additional adverse reactions in healthy volunteers receiving mesna alone included abdominal pain/colic, epigastric pain/burning, mucosal irritation, lightheadedness, back pain, arthralgia, myalgia, conjunctivitis, nasal congestion, rigors, paresthesia, photophobia, fatigue, lymphadenopathy, extremity pain, malaise, chest pain, dysuria, pleuritic pain, dry mouth, dyspnea, and hyperhidrosis. In healthy volunteers, mesna was commonly associated with a rapid (within 24 hours) decrease in lymphocyte count, which was generally reversible within one week of administration.
Because mesna is used in combination with ifosfamide or ifosfamide-containing chemotherapy regimens, it is difficult to distinguish the adverse reactions which may be due to mesna from those caused by the concomitantly administered cytotoxic agents.
Adverse reactions reasonably associated with mesna administered orally in four controlled studies in which patients received ifosfamide or ifosfamide-containing regimens are presented in Table 3.
Table 3: Adverse Reactions in ≥5% of Patients Receiving Mesna in combination with Ifosfamide-containing Regimens - * Intravenous dosing of ifosfamide and MESNEX followed by oral doses of mesna according to the applicable dosage schedule [see Dosage and Administration (2)].
Mesna Regimen
Intravenous-Oral-Oral* N exposed
119 (100%)
Incidence of AEs
106 (89.1%)
Nausea
64 (53.8)
Vomiting
45 (37.8)
Constipation
21 (17.6)
Leukopenia
21 (17.6)
Fatigue
24 (20.2)
Fever
18 (15.1)
Anorexia
19 (16.0)
Thrombocytopenia
16 (13.4)
Anemia
21 (17.6)
Granulocytopenia
15 (12.6)
Asthenia
21 (17.6)
Abdominal Pain
18 (15.1)
Alopecia
13 (10.9)
Dyspnea
11 (9.2)
Chest Pain
11 (9.2)
Hypokalemia
11 (9.2)
Diarrhea
17 (14.3)
Dizziness
5 (4.2)
Headache
13 (10.9)
Pain
10 (8.4)
Sweating Increased
2 (1.7)
Back Pain
6 (5.0)
Hematuria
7 (5.9)
Injection Site Reaction
10 (8.4)
Edema
9 (7.6)
Edema Peripheral
8 (6.7)
Somnolence
12 (10.1)
Anxiety
4 (3.4)
Confusion
6 (5.0)
Face Edema
5 (4.2)
Insomnia
11 (9.2)
Coughing
10 (8.4)
Dyspepsia
6 (5.0)
Hypotension
6 (5.0)
Pallor
6 (5.0)
Dehydration
7 (5.9)
Pneumonia
8 (6.7)
Tachycardia
7 (5.9)
Flushing
6 (5.0)
6.2 Postmarketing Experience
The following adverse reactions have been reported in the postmarketing experience of patients receiving mesna in combination with ifosfamide or similar drugs, making it difficult to distinguish the adverse reactions which may be due to mesna from those caused by the concomitantly administered cytotoxic agents. Because these reactions are reported from a population of unknown size, precise estimates of frequency cannot be made.
Cardiovascular: Hypertension
Gastrointestinal: Dysgeusia
Hepatobiliary: Hepatitis
Nervous System: Convulsion
Respiratory: Hemoptysis
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Mesna is used in combination with ifosfamide or other cytotoxic agents. Ifosfamide can cause fetal harm when administered to a pregnant woman. Refer to the ifosfamide prescribing information for more information on use during pregnancy.
MESNEX injection contains the preservative benzyl alcohol. Because benzyl alcohol is rapidly metabolized by a pregnant woman, benzyl alcohol exposure in the fetus is unlikely [see Warnings and Precautions (5.3) and Use in Specific Populations (8.4)].
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Mesna is used in combination with ifosfamide or other cytotoxic agents. Ifosfamide can cause fetal harm including embryo-fetal lethality. Refer to the ifosfamide prescribing information for more information on use during pregnancy.
In embryo-fetal development studies, oral administration of mesna to pregnant rats (500, 1000, 1500, and 2000 mg/kg) and rabbits (500 and 1000 mg/kg) during the period of organogenesis revealed no adverse developmental outcomes at doses approximately 10 times the maximum recommended total daily human equivalent dose based on body surface area.
8.2 Lactation
Risk Summary
Mesna is used in combination with ifosfamide or other cytotoxic agents. Ifosfamide is excreted in breast milk. Refer to the ifosfamide prescribing information for more information on use during lactation. There are no data on the presence of mesna in human or animal milk, the effect on the breastfed child, or the effect on milk production.
MESNEX injection contains the preservative benzyl alcohol. Because benzyl alcohol is rapidly metabolized by a lactating woman, benzyl alcohol exposure in the breastfed infant is unlikely. However, adverse reactions have occurred in premature neonates and low birth weight infants who received intravenously administered benzyl alcohol-containing drugs [see Warnings and Precautions (5.3) and Use in Specific Populations (8.4)].
Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment and for 1 week after the last dose of mesna or ifosfamide.
8.3 Females and Males of Reproductive Potential
Mesna is used in combination with ifosfamide or other cytotoxic agents. Ifosfamide can cause fetal harm when administered to a pregnant woman. Refer to the ifosfamide prescribing information for more information on contraception and effects on fertility.
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiation of mesna in combination with ifosfamide.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with mesna in combination with ifosfamide and for 6 months after the last dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with mesna in combination with ifosfamide and for 3 months after the last dose.
8.4 Pediatric Use
MESNEX injection contains the preservative benzyl alcohol which has been associated with serious adverse reactions and death when administered intravenously to premature neonates and low birth weight infants. Avoid use of MESNEX injection in premature neonates and low-birth weight infants [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of mesna did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. The ratio of ifosfamide to mesna should remain unchanged.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Mesna, USP is a detoxifying agent to inhibit the hemorrhagic cystitis induced by ifosfamide. The active ingredient mesna, USP is a synthetic sulfhydryl compound designated as sodium-2-mercaptoethane sulfonate with a molecular formula of C2H5NaO3S2 and a molecular weight of 164.18. Its structural formula is as follows:
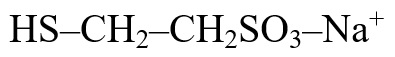
Mesna tablets USP are white to off white, modified capsule shaped, biconvex film-coated tablets, scored on one side and debossed with “I354” on the other side. They contain 400 mg mesna, USP. The excipients are lactose monohydrate, dibasic calcium phosphate, cornstarch, povidone, microcrystalline cellulose, magnesium stearate, hydroxypropyl methylcellulose, polyethylene glycol, and titanium dioxide.
FDA approved dissolution test specifications differ from USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mesna reacts chemically with the urotoxic ifosfamide metabolites, acrolein and 4-hydroxy-ifosfamide, resulting in their detoxification. The first step in the detoxification process is the binding of mesna to 4-hydroxy- ifosfamide forming a non-urotoxic 4-sulfoethylthioifosfamide. Mesna also binds to the double bonds of acrolein and to other urotoxic metabolites and inhibits their effects on the bladder.
12.3 Pharmacokinetics
Absorption
Following oral administration, peak plasma concentrations were reached within 1.5 to 4 hours and 3 to 7 hours for free mesna and total mesna (mesna plus dimesna and mixed disulfides), respectively. Oral bioavailability averaged 58% (range 45 to 71%) for free mesna and 89% (range 74 to 104%) for total mesna based on plasma AUC data from 8 healthy volunteers who received 1200 mg oral or intravenous doses.
Food does not affect the urinary availability of orally administered mesna.
Distribution
Mean apparent volume of distribution (Vd) for mesna is 0.652 ± 0.242 L/kg after intravenous administration which suggests distribution to total body water (plasma, extracellular fluid, and intracellular water).
Metabolism
Analogous to the physiological cysteine-cystine system, mesna is rapidly oxidized to its major metabolite, mesna disulfide (dimesna). Plasma concentrations of mesna exceed those of dimesna after oral or intravenous administration.
Excretion
Following intravenous administration of a single 800 mg dose, approximately 32% and 33% of the administered dose was eliminated in the urine in 24 hours as mesna and dimesna, respectively. Mean plasma elimination half- lives of mesna and dimesna are 0.36 hours and 1.17 hours, respectively. Mesna has a plasma clearance of 1.23 L/h/kg.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of mesna.
Mesna was not genotoxic in the in vitro Ames bacterial mutagenicity assay, the in vitro mammalian lymphocyte chromosomal aberration assay or the in vivo mouse micronucleus assay.
No studies on male or female fertility were conducted. No signs of male or female reproductive organ toxicity were seen in 6-month oral rat studies (≤ 2000 mg/kg/day) or 29-week oral dog studies (520 mg/kg/day) at doses approximately 10-fold higher than the maximum recommended human dose on a body surface area basis.
-
14 CLINICAL STUDIES
14.2 Oral Mesna
Clinical studies comparing recommended intravenous and oral mesna dosing regimens demonstrated incidences of grade 3 to 4 hematuria of <5%. Study 7 was an open label, randomized, two-way crossover study comparing three intravenous doses with an initial intravenous dose followed by two oral doses of mesna in patients with cancer treated with ifosfamide at a dose of 1.2 g/m2 to 2.0 g/m2 for 3 to 5 days. Study 8 was a randomized, multicenter study in cancer patients receiving ifosfamide at 2.0 g/m2 for 5 days. In both studies, development of grade 3 or 4 hematuria was the primary efficacy endpoint. The percent of patients developing hematuria in each of these studies is presented in Table 5.
Table 5. Percent of Mesna Patients Developing Grade 3 or 4 Hematuria Mesna Dosing Regimen
Study
Standard Intravenous
Regimen(number of patients)
Intravenous +
Oral Regimen(number of patients)
Study 7
0% (0/30)
3.6% (1/28)
Study 8
3.7% (1/27)
4.3% (1/23)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
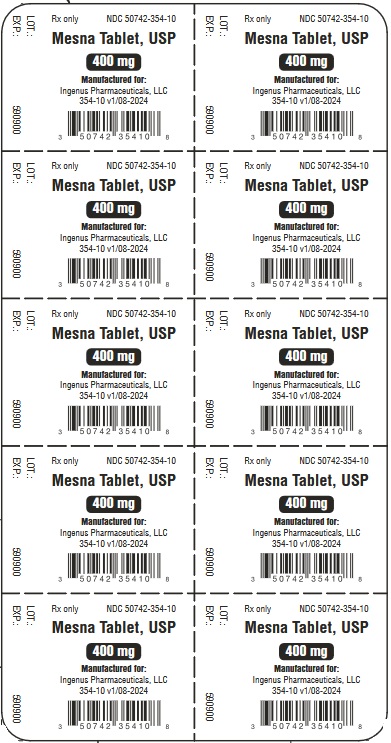
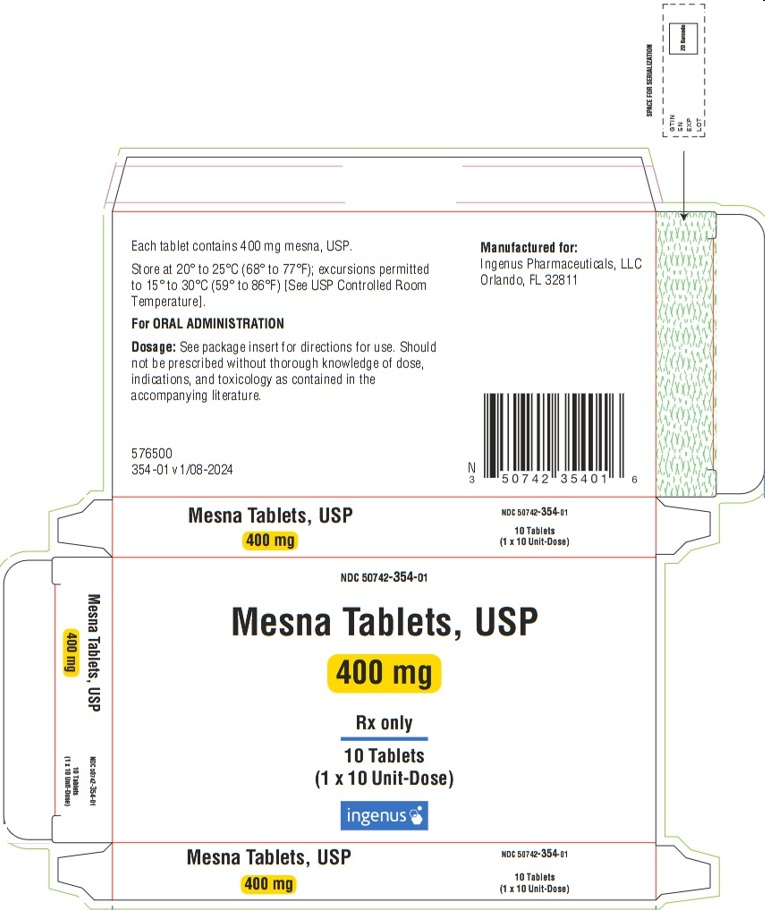
Mesna tablets, USP
- NDC: 50742-354-01
1 carton containing 1 blister card of 10 scored tablets.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Hypersensitivity
- Advise the patient to discontinue mesna and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction, including systemic anaphylactic reactions occur [see Warnings and Precautions (5.1)].
Dosing Instructions
- Advise the patient to take mesna at the exact time and in the exact amount as prescribed. Advise the patient to contact their healthcare provider if they vomit within 2 hours of taking oral mesna, or if they miss a dose of oral mesna [see Dosage and Administration (2.2)].
Hemorrhagic Cystitis
- Mesna does not prevent hemorrhagic cystitis in all patients nor does it prevent or alleviate any of the other adverse reactions or toxicities associated with ifosfamide. Advise the patient to report to their healthcare provider if his/her urine has turned a pink or red color [see Dosage and Administration (2.3)].
- Advise the patient to drink 1 to 2 liters of fluid each day during mesna therapy [see Dosage and Administration (2.3)].
Dermatologic Toxicity
- Advise the patient that Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic symptoms and bullous and ulcerative skin and mucosal reactions have occurred with mesna. Advise the patient to report to their healthcare provider if signs and symptoms of these syndromes occur [see Warnings and Precautions (5.2)].
Benzyl Alcohol Toxicity
- Advise patients that serious adverse reactions are associated with the benzyl alcohol found in mesna and other medications in premature neonates and low-birth weight infants [see Warnings and Precautions (5.3) and Use in Specific Populations (8.4)].
Embryo-Fetal Toxicity
- Mesna is used in combination with ifosfamide. Ifosfamide or other cytotoxic agents can cause fetal harm when administered to a pregnant woman. Inform female patients of the risk to a fetus and potential loss of the pregnancy. Advise females to inform their healthcare provider if they are pregnant or become pregnant [see Use in Specific Populations (8.1)].
Contraception
- Advise females of reproductive potential to use effective contraception during treatment with mesna in combination with ifosfamide and for 6 months after the last dose [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with mesna in combination with ifosfamide and for 3 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
- Advise lactating women not to breastfeed during treatment with mesna or ifosfamide and for 1 week after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
Mesna (mes' na)
tabletsWhat is the most important information I should know about mesna?
Mesna can cause serious allergic reactions and skin reactions. These serious reactions can happen the first time you are treated with mesna or after several months of treatment with mesna. Stop treatment with mesna and go to the nearest hospital emergency room right away if you develop any of the symptoms listed below:
- fever
- swelling of your face, lips, mouth, or tongue
- trouble breathing or wheezing
- itching
- burning
- skin rash or hives
- skin redness or swelling
- skin blisters or peeling
- feel lightheaded or faint
- feel like your heart is racing
- nausea
- vomiting
- joint or muscle aches
- mouth sores
See “What are the possible side effects of mesna?” for more information about side effects.
What is mesna?
Mesna is a prescription medicine used to reduce the risk of inflammation and bleeding of the bladder (hemorrhagic cystitis) in people who receive ifosfamide (a medicine used to treat cancer).
Mesna is not for use to reduce the risk of blood in the urine (hematuria) due to other medical conditions.
Do not take Mesna tablets if you are allergic to mesna or any of the ingredients in mesna tablets. See the end of this leaflet for a complete list of ingredients in mesna tablets.
Before you take mesna, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to any medicines
- are pregnant or plan to become pregnant.
Females who are able to become pregnant:
- Your healthcare provider will verify if you are pregnant or not before you start treatment with mesna and ifosfamide.
- You should use effective birth control (contraception) during treatment with mesna and ifosfamide and for 6 months after the last dose.
- Tell your healthcare provider if you become pregnant during treatment with mesna and ifosfamide.
Males with female partners who are able to become pregnant should use effective birth control during treatment with mesna and ifosfamide and for 3 months after the last dose.
You should also read the ifosfamide Prescribing Information for important pregnancy, contraception, and infertility information.
- are breastfeeding or plan to breastfeed. It is not known if mesna passes into your breast milk. Do not breastfeed during treatment and for 1 week after the last dose of mesna or ifosfamide.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive mesna?
- Mesna is given on the same day that you receive ifosfamide.
- Mesna can be given by an intravenous (IV) infusion into a vein or tablets taken by mouth.
- You will receive Mesna in one of two ways:
- Mesna intravenous (IV) infusion into a vein at the time you receive ifosfamide and 4 and 8 hours after you receive ifosfamide, OR
- Mesna intravenous (IV) infusion into a vein at the time you receive ifosfamide and mesna tablets taken by mouth 2 and 6 hours after you receive ifosfamide.
- Take mesna tablets at the exact times and the exact dose your healthcare provider tells you to take it.
- During treatment with mesna tablets, you should drink 4 to 8 cups of liquid (1 to 2 liters) each day.
- Tell your healthcare provider if you:
- vomit within 2 hours of taking mesna tablets by mouth
- miss a dose of mesna tablets
- have pink or red colored urine
What are the possible side effects of mesna?
Mesna may cause serious side effects, including:
See “What is the most important information I should know about mesna?”
- Mesna that is given by intravenous (IV) infusion contains the preservative benzyl alcohol. Benzyl alcohol has been shown to cause serious side effects and death in premature newborns and low-birth weight babies. Avoid use of MESNEX injection in premature newborns and low birth weight infants. Mesna tablets do not contain benzyl alcohol.
The most common side effects of mesna when given with ifosfamide include:
- nausea
- vomiting
- constipation
- decreased white blood cell count
- tiredness
- fever
- decreased appetite
- decreased platelet count
- decreased red blood cell count
- diarrhea
- weakness
- stomach (abdomen) pain
- headache
- hair loss
- sleepiness
These are not all the possible side effects of mesna.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
How should I store mesna tablets?
- Store mesna tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep mesna tablets and all medicines out of the reach of children.
General information about the safe and effective use of mesna.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use mesna for a condition for which it was not prescribed. Do not give mesna to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about mesna that is written for health professionals.
What are the ingredients in mesna tablets?
Active ingredient: Mesna, USP
Inactive ingredients:
Mesna tablets, USP: lactose monohydrate, dibasic calcium phosphate, cornstarch, povidone, magnesium stearate, microcrystalline cellulose, polyethylene glycol, hydroxypropyl methylcellulose, and titanium dioxide.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Rx only
Mesnex is a registered trademark of Baxter International Inc.
For more information, call 1-877-748-1970.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 01/2025
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
- PACKAGE Carton - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MESNA
mesna tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50742-354 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESNA (UNII: NR7O1405Q9) (2-MERCAPTOETHANESULFONIC ACID - UNII:VHD28S0H7F) MESNA 400 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to off-white) Score 2 pieces Shape CAPSULE Size 16mm Flavor Imprint Code I354 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50742-354-01 1 in 1 CARTON 01/14/2025 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218871 01/14/2025 Labeler - Ingenus Pharmaceuticals, LLC (833250017) Registrant - RiconPharma LLC (859035318) Establishment Name Address ID/FEI Business Operations Ingenus Pharmaceuticals NJ, LLC 964680206 manufacture(50742-354)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.