ATTRUBY- acoramidis hydrochloride tablet, film coated
Attruby by
Drug Labeling and Warnings
Attruby by is a Prescription medication manufactured, distributed, or labeled by BridgeBio Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATTRUBY safely and effectively. See full prescribing information for ATTRUBY.
ATTRUBY™ (acoramidis) tablets, for oral administration,
Initial U.S. Approval: 2024INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
The recommended dosage of ATTRUBY is 712 mg orally twice daily. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 356 mg acoramidis (3)
CONTRAINDICATIONS
None. (4)
To report SUSPECTED ADVERSE REACTIONS, contact BridgeBio Pharma Inc. at 1-844-550-2246 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data reflect the exposure of 421 participants with ATTR-CM to ATTRUBY 712 mg (administered as two 356 mg tablets) administered orally twice daily in a randomized, double-blind, placebo-controlled trial of 30 months fixed treatment duration. The median duration of exposure to ATTRUBY in the safety population was 29 months. There was a higher frequency of gastrointestinal (GI) adverse reactions such as diarrhea 11.6% versus 7.6% and upper abdominal pain 5.5% versus 1.4% in the ATTRUBY versus placebo group, respectively. The majority of these GI adverse reactions were categorized as mild and resolved without drug discontinuation.
A similar proportion of ATTRUBY-treated and placebo-treated participants discontinued study drug because of an adverse event (9.3% and 8.5%, respectively).
Laboratory Tests
Increase in Serum Creatinine and Decrease in eGFR
Initiation of ATTRUBY causes an increase in serum creatinine and decrease in eGFR which generally occurs within 4 weeks of starting therapy and stabilizes. In a trial of adults with ATTR-CM, a mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was observed in the ATTRUBY and placebo groups, respectively, at Day 28. The changes in serum creatinine and eGFR were reversible after treatment discontinuation.
-
7 DRUG INTERACTIONS
UDP-glucuronosyltransferases (UGT) Inducers and Strong CYP3A Inducers
Acoramidis is metabolized by UGT enzyme-mediated glucuronidation. Concomitant use of UGT inducers can potentially decrease acoramidis exposure. While acoramidis is not metabolized by CYP3A, strong CYP3A inducers can also induce UGT enzymes. Avoid concomitant use of ATTRUBY with UGT inducers and strong CYP3A inducers.
Sensitive Cytochrome P450 2C9 (CYP2C9) substrates
Acoramidis inhibits CYP2C9 and may result in an increase in CYP2C9 substrate concentrations when these drugs are co administered. Consider more frequent monitoring of patients for evidence of increased exposure (for example, signs of exposure related toxicity) when ATTRUBY is co administered with sensitive CYP2C9 substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with acoramidis use in pregnant women are insufficient to establish a drug associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproductive studies in rats and rabbits, no embryofetal abnormalities were observed at exposures up to 34 times and 13 times the clinical exposure at the maximum recommended human dose, respectively (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report pregnancies to the BridgeBio reporting line at 1-844-550-2246.
Data
Animal Data
In pregnant rats, oral administration of acoramidis (0, 50, 350, and 1,000 mg/kg/day) throughout organogenesis did not result in any adverse effects on embryofetal development at up to 1,000 mg/kg/day, approximately 34 times the clinical exposure at the maximum recommended human dose (MRHD) based on AUC.
In pregnant rabbits, oral administration of acoramidis (0, 25, 75, and 200 mg/kg/day) throughout organogenesis resulted in increased pre-implantation loss at 200 mg/kg/day, a dose that caused maternal toxicity (26% reduced body weight gain). No embryofetal abnormalities were observed at 200 mg/kg/day, approximately 13 times the clinical exposure at the MRHD based on AUC.
In a pre- and postnatal developmental toxicity study, pregnant rats received oral administration of acoramidis at doses of 0, 50, 350, or 1,000 mg/kg/day throughout pregnancy and lactation (Gestation Day 6 to Lactation Day 20). Maternal death, body weight reduction, and decreased number of females with live born pups (due to increase in resorbed litters) were observed at 1,000 mg/kg/day, approximately 43 times the clinical exposure at the MRHD based on AUC. Decreased body weight gain from the neonatal period to weaning and learning deficits were observed in the offspring of dams given 1,000 mg/kg/day. No adverse effects on pre- and postnatal development were observed at 350 mg/kg/day, approximately 18 times the clinical exposure at the MRHD based on AUC.
8.2 Lactation
Risk Summary
There are no available data on the presence of acoramidis in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ATTRUBY and any potential adverse effects on the breastfed child from ATTRUBY or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ATTRUBY have not been established in pediatric patients.
8.5 Geriatric Use
No dosage adjustment is required for elderly patients (≥65 years) [see Clinical Pharmacology (12.3)]. Of the total number of participants randomized in the clinical study (n=632), 97% were 65 years and over, with a median age of 78 years.
- 10 OVERDOSAGE
-
11 DESCRIPTION
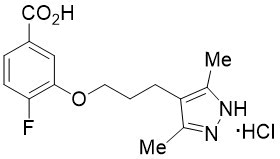
ATTRUBY contains 356 mg acoramidis equivalent to 400 mg acoramidis HCl.
Acoramidis HCl is a transthyretin stabilizer. The chemical name of acoramidis HCl is 3-[3-(3,5-dimethyl-1H-pyrazol-4-yl)propoxy]-4-fluorobenzoic acid hydrochloride. The molecular formula is C15H18FN2O3Cl, and the molecular weight is 328.77 g/mol. The structural formula is:
Acoramidis HCl is a white to tan solid. The solubility of acoramidis is ≥ 12 micrograms/mL from pH 1.2 to 6.8 in aqueous media.
ATTRUBY is supplied as a white, film-coated, oval tablet, contains 356 mg acoramidis, printed with the BridgeBio company logo followed by “ACOR” in black ink on one side.
The inactive ingredients are croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and silicon dioxide. The film coating and printing ink contain black iron oxide, glyceryl monocaprylocaprate, hypromellose, polyvinyl alcohol, propylene glycol, talc, titanium dioxide, and vinyl alcohol graft copolymer.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Acoramidis is a selective stabilizer of transthyretin (TTR). Acoramidis binds TTR at thyroxine binding sites and slows dissociation of the TTR tetramer into its constituent monomers, the rate-limiting step in amyloidogenesis.
12.2 Pharmacodynamics
TTR Stabilization
Changes in serum TTR level or in vitro TTR stabilization assays were utilized as pharmacodynamic markers of TTR stabilization. Increases in mean serum TTR levels were observed by Day 28 in ATTR-CM patients treated with ATTRUBY. Near-complete in vitro TTR stabilization was observed as early as Day 28 and through completion of a 30-month study of patients with ATTR-CM (wild-type and variant) treated with the recommended dosage [see Clinical Studies (14)].
Free thyroxine
ATTRUBY may decrease serum concentrations of free thyroxine without an accompanying change in thyroid stimulating hormone (TSH). A reduction in free thyroxine values has been observed with transthyretin stabilizers probably due to reduced thyroxine binding to or displacement from transthyretin (TTR).
NT-proBNP and Troponin I
In a clinical study of ATTRUBY in patients with ATTR-CM, at Month 30, the increase in N-terminal prohormone of brain natriuretic peptide [NT-proBNP] and troponin I was lower with ATTRUBY versus placebo. The increase in NT-proBNP at Month 30 for ATTRUBY was about half that of placebo [see Clinical Studies (14)].
12.3 Pharmacokinetics
The systemic exposures (Cmax and AUC) increase in a less than dose proportional manner following single and multiple doses of acoramidis. Over the dose range from 89 mg twice daily to 712 mg twice daily, AUC increases only 130%. Acoramidis steady state is achieved by 4 days with approximately 1.3-fold accumulation at the approved recommended dosage. At steady state, a dose of 712 mg twice daily results in a mean (SD) Cmax of 13700 (6090) ng/mL and AUC0-12h of 47200 (10300) ng.h/mL.
Absorption
The time to Cmax of acoramidis (Tmax) is approximately 1 hour following oral administration.
Distribution
The apparent steady-state volume of distribution for acoramidis is 654 liters. Acoramidis is 96% bound to human plasma proteins in vitro. Acoramidis primarily binds to TTR.
Elimination
The effective half-life of acoramidis is approximately 6 hours with a steady state apparent clearance of 16 L/hr.
Metabolism
Acoramidis is primarily metabolized by glucuronidation via UGT1A9, UGT1A1 and UGT2B7. Acoramidis-β-D-glucuronide (Acoramidis-AG) is the predominant metabolite of acoramidis (8% of total circulating drug related moieties).
Acoramidis-AG is approximately 1/3 as pharmacologically active compared with acoramidis, has a low potential for covalent binding, and does not contribute to pharmacological activity.
Excretion
After a single oral dose of radiolabeled acoramidis 712 mg to healthy adult subjects, approximately 32% of the dose radioactivity was recovered in feces (15% unchanged), and approximately 68% was recovered in urine (<10% unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of acoramidis were observed based on age, race/ethnicity (including Japanese and non-Japanese), sex, or renal impairment. The effect of hepatic impairment (Child Pugh A, B, or C) on acoramidis pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies
Following the administration of acoramidis (712 mg, BID) in a clinical study in healthy adult volunteers, there was not a clinically significant increase in exposure to the organic anion transporter-1 (OAT1) substrate (adefovir) and to OAT3 substrate (oseltamivir carboxylate).
Concomitant diuretic use in patients does not affect steady-state plasma acoramidis concentrations.
In Vitro Studies
Cytochrome P450 Enzymes:
Acoramidis is a time-dependent inhibitor of CYP2C9, but does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C19, CYP2D6, or CYP3A4/5. Acoramidis does not induce CYP1A2, CYP2B6, or CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
There was no evidence of increased incidence of neoplasia in a 2-year carcinogenicity study in male rats dosed up to 50 mg/kg and in female rats dosed up to 350 mg/kg, which provided exposures approximately equivalent to and 11 times the AUC at the maximum recommended human dose (MRHD), respectively. There was no evidence of an increased incidence of neoplasia in transgenic (Tg.rasH2) mice following repeated daily administration for 26 weeks at daily doses up to 300 mg/kg.
Mutagenesis
There was no evidence of mutagenicity or clastogenicity for acoramidis in an Ames assay or in vivo rat micronucleus and alkaline comet assay.
Impairment of Fertility
In a male and female fertility study, rats were orally administered acoramidis at 0, 50, 350, and 1,000 mg/kg/day. Male rats were given acoramidis prior to and during cohabitation for a total of up to 52 days. Female rats were given acoramidis prior to and during cohabitation until implantation of the embryo (Gestational Day 7) for a total of up to 34 days. There were no effects on fertility, reproductive performance, or mating behavior in male or female rats at doses up to 1,000 mg/kg/day, approximately 38-times the AUC at the MRHD.
-
14 CLINICAL STUDIES
The efficacy of ATTRUBY was demonstrated in a multicenter, international, randomized, double-blind, placebo-controlled study in 611 adult patients with wild-type or variant (hereditary or de novo) ATTR-CM (NCT03860935).
Participants were randomized (2:1) to receive ATTRUBY 712 mg (n=409) or placebo (n=202) twice daily for 30 months. Treatment assignment was stratified by type of ATTR-CM [variant (ATTRv-CM) or wild-type (ATTRwt-CM)], NT-proBNP level, and estimated glomerular filtration rate (eGFR). The mean age of study participants was 77 years, 90.8% were male, 87.9% were White, 4.7% Black or African American, 2.1% Asian, 5.3% race other, 19% had a history of permanent pacemaker and 58% had a history of atrial fibrillation. No significant imbalance in baseline characteristics was observed between the two treatment groups.
Participants were permitted to initiate open-label tafamidis after 12 months in the study. A total of 107 participants received tafamidis: 61 (14.9%) in the ATTRUBY arm and 46 (22.8%) in the placebo arm. The median time to initiation of tafamidis for these 107 participants was 17 months.
The primary composite endpoint included all-cause mortality (ACM) and cumulative frequency of cardiovascular-related hospitalizations (CVH) over 30 months, analyzed hierarchically using the stratified Finkelstein-Schoenfeld (F-S) test. The F-S test demonstrated a statistically significant reduction (p=0.018) in ACM and cumulative frequency of CVH in the ATTRUBY arm versus the placebo arm. All-cause mortality was reported in 19% and 26% of participants in the ATTRUBY and placebo groups, respectively. The majority (79%) of the deaths were cardiovascular. CVH was reported in 27% and 43% of participants in the ATTRUBY and placebo groups, respectively. The mean number of CVH events was 0.3 vs 0.6 per year. The majority (59%) of CVH were heart failure hospitalizations reported in 13% and 26% of the participants in the ATTRUBY and placebo groups, respectively.
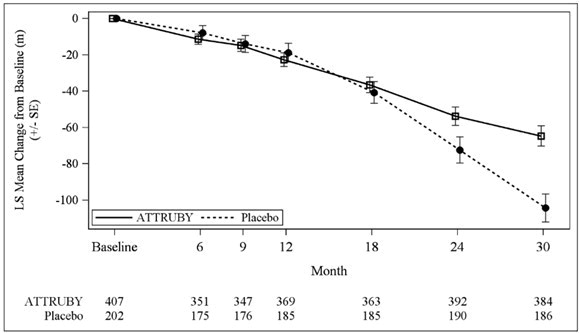
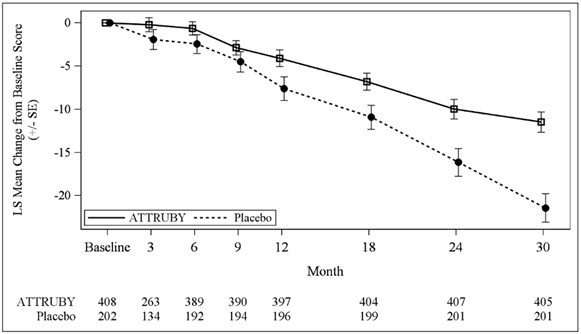
The treatment effect of ATTRUBY on functional capacity and health status was assessed by the 6MWD and the Kansas City Cardiomyopathy Questionnaire-Overall Summary score (KCCQ-OS) respectively. At month 30, the LS mean difference (95% CI) in change from baseline in 6MWD was 40 [21, 58] meters (p < 0.0001) and change from baseline in KCCQ-OS was 10 [6, 14] points (p < 0.0001) (Figure 1 and Figure 2).
Abbreviations: 6MWD = Six-Minute Walk Distance; KCCQ-OS = Kansas City Cardiomyopathy Questionnaire Overall Summary Score; SE = standard error; LS = least squares.
The changes from baseline in 6MWT and KCCQ-OS were analyzed using the mixed model for repeated measures (MMRM) with treatment group, visit, genotype (ATTRv-CM vs ATTRwt-CM), NT-proBNP level (≤ 3000 vs > 3000 pg/mL), eGFR level (≥ 45 vs < 45 mL/min/1.73 m2) and treatment group-by-visit interaction as factors, and baseline value as covariate.
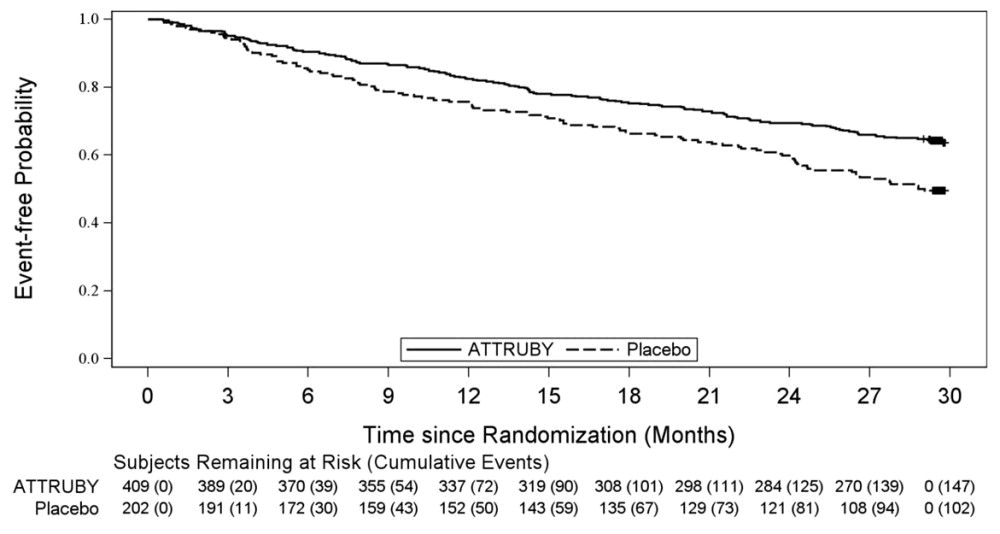
A Cox regression analysis indicated a 35.5% decrease in the risk of the composite of ACM or first CV hospitalization (hazard ratio: 0.645 [95% CI: 0.500, 0.832]). A Kaplan-Meier plot of time to first event of ACM or CVH is shown in Figure 3.
Figure 3: Time to First All-cause Mortality or Cardiovascular-Related Hospitalization over Month 30, mITT Population
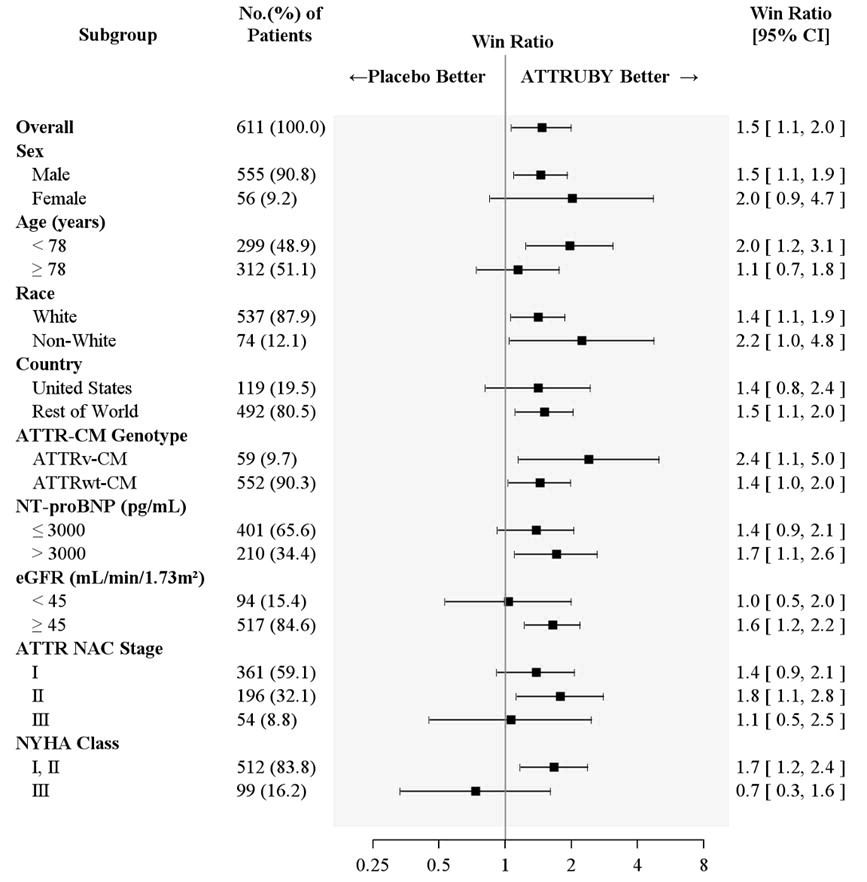
Figure 4 shows the treatment effects by prespecified subgroups.
Figure 4: Win-Ratio Analyses for Hierarchical Combination of All-Cause Mortality and Cardiovascular-Related Hospitalization by Overall and Subgroup, mITT Population
Abbreviations: ATTR-CM = transthyretin amyloid cardiomyopathy; ATTRv-CM = variant ATTR-CM; ATTRwt-CM = wild-type ATTR-CM; eGFR = estimated glomerular filtration rate; mITT = modified intent-to-treat; NAC = National Amyloidosis Centre; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; NYHA = New York Heart Association
All-Cause Mortality includes heart transplant, CMAD and all-cause death. Cardiovascular-related hospitalizations include cardiovascular hospitalizations and urgent unplanned visits requiring treatment with intravenous diuretic for decompensated heart failure. Non-White includes American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, Other, multiple races and Not reported.
ATTR NAC Stage: ATTR Stage I, defined as NT-proBNP ≤ 3000 ng/L and eGFR ≥ 45 mL/min/1.73 m2; Stage III, defined as NT-proBNP > 3000 ng/L and eGFR < 45 mL/min/1.73 m2, the remainder categorized as Stage II. -
16 HOW SUPPLIED/STORAGE AND HANDLING
ATTRUBY (acoramidis) tablets, 356 mg, are white, film-coated, oval tablets, printed with the BridgeBio company logo followed by “ACOR” in black ink on one side.
ATTRUBY tablets are supplied as a carton of 112 tablets: 4 blister cards (each containing 28 tablets)– (NDC: 82228-712-28).
Store ATTRUBY at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Store in original blister card until use to protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy
Advise patients who are exposed to ATTRUBY during pregnancy to contact the BridgeBio reporting line at 1-844-550-2246. Advise patients to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
For more information about ATTRUBY, go to www.ATTRUBY.com
Distributed by:
BridgeBio Pharma, Inc.
Palo Alto, CA 94304 -
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 11/2024
PATIENT INFORMATION
ATTRUBYTM (ah-troo-be)
(acoramidis)
tabletsWhat is ATTRUBY?
ATTRUBY is a prescription medicine used to treat adults with a disease that affects the heart muscle called cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM), to reduce death and hospitalization related to heart problems.
It is not known if ATTRUBY is safe and effective in children.Before taking ATTRUBY, tell your healthcare provider about all your medical conditions, including if you: - are pregnant or plan to become pregnant. It is not known if ATTRUBY will harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ATTRUBY. You may also report your pregnancy by calling the BridgeBio reporting line at 1-844-550-2246.
- are breastfeeding or plan to breastfeed. It is not known if ATTRUBY passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with ATTRUBY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How should I take ATTRUBY?
- Take ATTRUBY exactly as your healthcare provider tells you to.
- Take ATTRUBY tablets by mouth 2 times a day, with or without food.
- Swallow tablets whole. Do not cut, crush, or chew tablets.
What are the possible side effects of ATTRUBY?
The most common side effects of ATTRUBY were mild and include:
- stomach-area (abdominal) pain
- diarrhea
These are not all of the possible side effects of ATTRUBY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ATTRUBY?
- Store ATTRUBY tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store in original blister card until use to protect from moisture.
Keep ATTRUBY and all medicines out of the reach of children. General information about the safe and effective use of ATTRUBY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ATTRUBY for a condition for which it was not prescribed. Do not give ATTRUBY to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ATTRUBY that is written for health professionals.What are the ingredients in ATTRUBY?
Active ingredient: acoramidis
Inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and silicon dioxide. The film coating and printing ink contain black iron oxide, glyceryl monocaprylocaprate, hypromellose, polyvinyl alcohol, propylene glycol, talc, titanium dioxide, and vinyl alcohol graft copolymer.
Distributed by: BridgeBio Pharma, Inc., Palo Alto, CA 94304
For more information about ATTRUBY, go to www.ATTRUBY.com or call 1-844-550-2246
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 356 mg Carton Label
NDC: 82228-712-28
Rx only
bridgebio
Attruby™
(acoramidis) tablets
356 mg per tablet
Take two 356 mg tablets two times a day
(712 mg dose two times a day)Four 7-day blister cards
with 28 tablets per card
(112 tablets) -
INGREDIENTS AND APPEARANCE
ATTRUBY
acoramidis hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82228-712 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength acoramidis hydrochloride (UNII: VY9C88C2NV) (acoramidis - UNII:T12B44A1OE) acoramidis 356 mg Inactive Ingredients Ingredient Name Strength Microcrystalline Cellulose (UNII: OP1R32D61U) Croscarmellose Sodium (UNII: M28OL1HH48) Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) POLYVINYL ALCOHOL GRAFT POLYETHYLENE GLYCOL COPOLYMER (3:1; 45000 MW) (UNII: 23ZQ42JZZH) Talc (UNII: 7SEV7J4R1U) Titanium Dioxide (UNII: 15FIX9V2JP) Glyceryl Mono- And Dicaprylocaprate (UNII: U72Q2I8C85) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) Ferrosoferric Oxide (UNII: XM0M87F357) Propylene Glycol (UNII: 6DC9Q167V3) Hypromellose 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color white (white) Score no score Shape OVAL (OVAL) Size 15mm Flavor Imprint Code ;ACOR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82228-712-28 4 in 1 CARTON 11/22/2024 1 1 in 1 DOSE PACK 1 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216540 11/22/2024 Labeler - BridgeBio Pharma, Inc. (117109644)
Trademark Results [Attruby]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ATTRUBY 97288153 not registered Live/Pending |
BridgeBio Pharma, Inc. 2022-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.