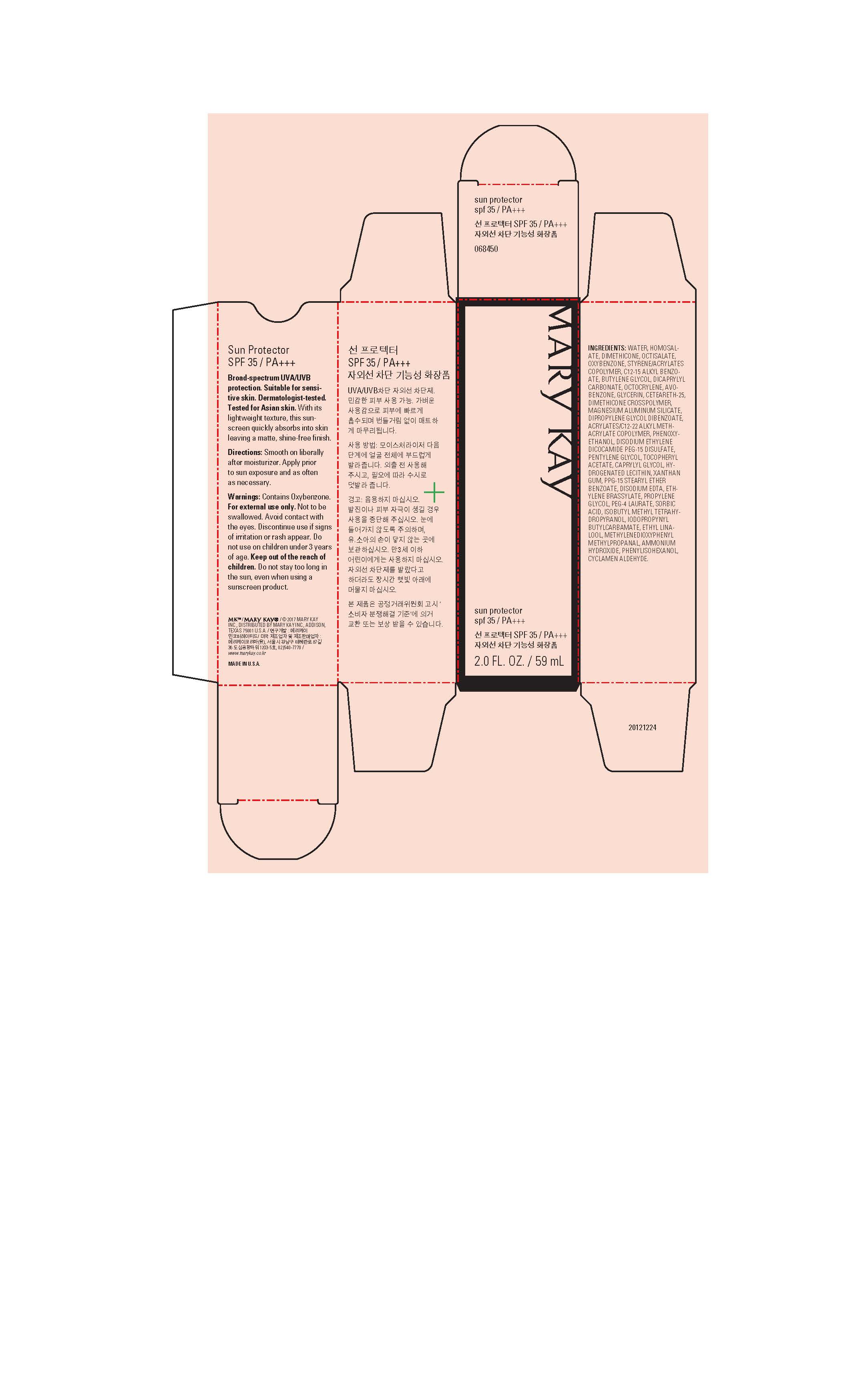
MARY KAY SUN PROTECTOR SPF 35- avobenzone, homosalate, octisalate, oxybenzone cream
Mary Kay Sun Protector SPF 35 by
Drug Labeling and Warnings
Mary Kay Sun Protector SPF 35 by is a Otc medication manufactured, distributed, or labeled by Mary Kay Inc., Le Papillon, Ltd., Englewood Lab, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PURPOSE
- INDICATIONS & USAGE
- Warnings
- Directions
-
INGREDIENTS
WATER, HOMOSALATE, DIMETHICONE, OCTISALATE, OXYBENZONE, STYRENE/ACRYLATES
COPOLYMER, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, DICAPRYLYL
CARBONATE, OCTOCRYLENE, AVOBENZONE, GLYCERIN, CETEARETH-25, DIMETHICONE CROSSPOLYMER,
MAGNESIUM ALUMINUM SILICATE, DIPROPYLENE GLYCOL DIBENZOATE,
ACRYLATES/C12-22 ALKYL METHACRYLATE COPOLYMER, PHENOXYETHANOL, DISODIUM ETHYLENE
DICOCAMIDE PEG-15 DISULFATE, PENTYLENE GLYCOL, TOCOPHERYL
ACETATE, CAPRYLYL GLYCOL, HYDROGENATED LECITHIN, XANTHAN GUM, PPG-15 STEARYL ETHER
BENZOATE, DISODIUM EDTA, ETHYLENE BRASSYLATE, PROPYLENE GLYCOL, PEG-4 LAURATE, SORBIC
ACID, ISOBUTYL METHYL TETRAHYDROPYRANOL, IODOPROPYNYL BUTYLCARBAMATE, ETHYL LINALOOL, METHYLENEDIOXYPHENYL METHYLPROPANAL, AMMONIUM HYDROXIDE, PHENYLISOHEXANOL, CYCLAMEN ALDEHYDE. - Principal Display Panel - 59 mL carton
-
INGREDIENTS AND APPEARANCE
MARY KAY SUN PROTECTOR SPF 35
avobenzone, homosalate, octisalate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51531-8450 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) GLYCERIN (UNII: PDC6A3C0OX) CETEARETH-25 (UNII: 8FA93U5T67) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PENTYLENE GLYCOL (UNII: 50C1307PZG) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) XANTHAN GUM (UNII: TTV12P4NEE) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) PEG-4 LAURATE (UNII: AYF4VM3N1Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBIC ACID (UNII: X045WJ989B) 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) ETHYL LINALOOL (UNII: SF2JS9GF5T) 3-(3,4-METHYLENEDIOXYPHENYL)-2-METHYLPROPANAL (UNII: L65EG8H6PA) PHENYLISOHEXANOL (UNII: M56178H183) CYCLAMEN ALDEHYDE (UNII: 4U37UX0E1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51531-8450-2 1 in 1 CARTON 06/26/2013 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 51531-8450-0 0.49 mL in 1 PACKET; Type 0: Not a Combination Product 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 06/26/2013 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-8450) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 081158134 manufacture(51531-8450) Establishment Name Address ID/FEI Business Operations Englewood Lab, Inc. 080987545 pack(51531-8450) Establishment Name Address ID/FEI Business Operations Le Papillon, Ltd. 040070823 pack(51531-8450)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.