AMTAGVI- lifileucel suspension
AMTAGVI by
Drug Labeling and Warnings
AMTAGVI by is a Other medication manufactured, distributed, or labeled by Iovance Biotherapeutics Inc., Iovance Biotherpeutics Manufacturing, LLC, WuXi Advanced Therapies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AMTAGVI safely and effectively. See full prescribing information for AMTAGVI.
AMTAGVI (lifileucel) suspension for intravenous infusion
Initial U.S. Approval: 2024WARNING: TREATMENT-RELATED MORTALITY, PROLONGED SEVERE CYTOPENIA, SEVERE INFECTION, CARDIOPULMONARY and RENAL IMPAIRMENT
See full prescribing information for complete boxed warning.
- Monitor patients for prolonged severe cytopenia and monitor for internal organ hemorrhage (5.1, 5.2, 5.3)
- Treat severe infections (5.1, 5.4)
- Monitor cardiopulmonary and renal functions throughout the treatment course (5.1, 5.5, 5.6, 5.7)
Administer in an inpatient hospital setting. An intensive care facility and specialists skilled in cardiopulmonary or intensive care medicine must be available (2.1, 6.1)
INDICATIONS AND USAGE
AMTAGVI is a tumor-derived autologous T cell immunotherapy indicated for the treatment of adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor. This indication is approved under accelerated approval based on objective response rate (ORR). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s) (1)
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
Verify the patient's identity prior to infusion.
- Administer AMTAGVI in an inpatient hospital setting with an intensive care facility (2.1)
- The AMTAGVI dose is between 7.5 × 109 and 72 × 109 viable cells (2.1)
- Administer a lymphodepleting regimen before infusion of AMTAGVI (2.2)
- Do NOT use a leukocyte depleting filter with AMTAGVI (2.2)
- Premedicate the patient with acetaminophen, or equivalent, and diphenhydramine, or another H1-antihistamine (2.2)
- Avoid prophylactic use of systemic corticosteroids (2.2)
- Administer entire dose of AMTAGVI (2.2)
- Administer IL-2 (aldesleukin) after infusion of AMTAGVI (2.2)
- See Full Prescribing Information for instructions on receipt, preparation, and administration of AMTAGVI (2.2, 16)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity reactions: Monitor for hypersensitivity reactions during infusion (5.8)
ADVERSE REACTIONS
The most common (incidence of greater than or equal to 20%) non-laboratory adverse reactions in order of decreasing frequency were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash, hypotension, alopecia, infection, hypoxia, and dyspnea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Iovance Biotherapeutics, Inc. at 1-833-400-IOVA (4682) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: TREATMENT-RELATED MORTALITY, PROLONGED SEVERE CYTOPENIA, SEVERE INFECTION, CARDIOPULMONARY and RENAL IMPAIRMENT
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Treatment-Related Mortality
5.2 Prolonged Severe Cytopenia
5.3 Internal Organ Hemorrhage
5.4 Severe Infection
5.5 Cardiac Disorder
5.6 Respiratory Failure
5.7 Acute Renal Failure
5.8 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: TREATMENT-RELATED MORTALITY, PROLONGED SEVERE CYTOPENIA, SEVERE INFECTION, CARDIOPULMONARY and RENAL IMPAIRMENT
- Monitor patients for prolonged severe cytopenia and monitor for internal organ hemorrhage [see Warnings and Precautions (5.1, 5.2, 5.3)].
- Administer filgrastim or a biosimilar product to patients beginning Day 1 after AMTAGVI and continuing daily until the absolute neutrophil count (ANC) is greater than 1000 per mm3 for 3 consecutive days, or per institutional standard.
- Treat severe infections [see Warnings and Precautions (5.1, 5.4)].
- Monitor cardiopulmonary and renal functions throughout the treatment course. [see Warnings and Precautions (5.1, 5.5, 5.6, 5.7)].
Administer in an inpatient hospital setting. An intensive care facility and specialists skilled in cardiopulmonary or intensive care medicine must be available [see Dosage and Administration (2.1), and Adverse Reactions (6.1)].
-
1 INDICATIONS AND USAGE
AMTAGVI is a tumor-derived autologous T cell immunotherapy indicated for the treatment of adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor.
This indication is approved under accelerated approval based on objective response rate (ORR) [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
Verify the patient's identity prior to infusion.
2.1 Dose
Administer in an inpatient hospital setting under the supervision of a physician experienced in the use of anticancer agents. An intensive care facility and specialists skilled in cardiopulmonary or intensive care medicine must be available.
AMTAGVI is provided as a single dose for infusion containing a suspension of tumor-derived T cells. The dose is supplied in 1 to 4 patient-specific IV infusion bag(s) in individual protective metal cassettes. Each dose contains 7.5 × 109 to 72 × 109 viable cells.
2.2 Administration
AMTAGVI is for autologous use only.
The patient's identity must match the patient identifiers on the AMTAGVI cassette(s) and infusion bag(s).
Preparing Patient for AMTAGVI Infusion
Confirm availability of AMTAGVI and IL-2 (aldesleukin) prior to starting the lymphodepleting regimen.
Pretreatment
Administer a lymphodepleting chemotherapy regimen of cyclophosphamide 60 mg/kg intravenously with mesna daily for 2 days followed by fludarabine 25 mg/m2 intravenously daily for 5 days before infusion of AMTAGVI.
Infuse AMTAGVI as soon as possible after 24 hours have elapsed following the last dose of fludarabine, but no later than 4 days.
Receipt of AMTAGVI
AMTAGVI is shipped directly to the treatment center in the vapor phase of a liquid nitrogen cryoshipper. All treatment centers should have onsite storage in vapor phase of liquid nitrogen.
Product and patient-specific labels are located on both the product infusion bag(s) and protective metal cassette(s), which are inside the liquid nitrogen cryoshipper.
- Match the identity of the patient with the patient identifiers on the cassette(s) and infusion bag(s) upon receipt.
- Confirm the number of AMTAGVI cassette(s) and infusion bag(s) matches the total number of cassettes and infusion bags on the shipment packing slip.
- Store AMTAGVI frozen in the vapor phase of liquid nitrogen (less than or equal to minus 150°C).
Administration of AMTAGVI
The AMTAGVI dose is contained in 1 to 4 cryopreserved patient-specific infusion bag(s) in individual protective metal cassette(s). Thaw and infuse 1 bag at a time if more than 1 bag has been provided. Wait to thaw the next bag until the previous bag has been safely and completely administered.
Preparation of AMTAGVI
Do not thaw the product until it is ready to be infused. Coordinate the timing of AMTAGVI thaw and infusion. Confirm the infusion time in advance and adjust the start time for thaw so that AMTAGVI is available for infusion when the patient is ready. Once 1 bag of AMTAGVI is thawed, the infusion should be started as soon as possible and must be completed within 3 hours at room or ambient temperature (18°C to 25°C).
- 1. Confirm the availability of IL-2 (aldesleukin).
- 2. Prior to AMTAGVI preparation, match the recipient's identity with the patient identifiers on the AMTAGVI cassette label. Do not remove the AMTAGVI infusion bag from the cassette if the patient identifiers on the AMTAGVI cassette label do not match the intended patient. Contact Iovance Biotherapeutics, Inc. at 1-833-400-IOVA if there are any discrepancies.
- 3. Once recipient identification on the cassette is confirmed, remove the AMTAGVI infusion bag from the cassette. Check that the patient identifiers on the cassette label match the patient identifiers on the AMTAGVI infusion bag label and match the recipient's identity with the patient identifiers on the AMTAGVI infusion bag label. Contact Iovance Biotherapeutics, Inc. at 1-833-400-IOVA if there are any discrepancies.
- 4. Inspect each bag for any breaks or cracks prior to thawing. Inspect the spike ports for any damage prior to thawing. If a bag is damaged or compromised, do not infuse the contents and contact Iovance Biotherapeutics, Inc. at 1-833-400-IOVA.
- 5. For thawing, place the infusion bag inside a second sealable bag (preferably sterile) per local guidelines in case of a leak and to protect ports from contamination.
- 6. Thaw AMTAGVI at approximately 35°C to 39°C using either a water bath or a dry thaw method until there is no visible ice or frozen contents in the infusion bag. Total time from start of thaw until completion of thawing should be no more than 10 minutes.
- 7. Immediately remove bag from thawing device. Remove the infusion bag from the sealable plastic bag and wipe dry. Do not wash, spin down, or resuspend AMTAGVI in new media prior to infusion.
- 8. Once thawed, administer each bag of AMTAGVI as soon as possible. If needed, AMTAGVI may be maintained at room temperature (18°C to 25°C) not to exceed 3 hours. Do not re-freeze or refrigerate thawed product.
- 9. Prior to infusion, inspect the contents of the thawed infusion bag. If cell clumps are visible, gently mix the contents of the bag by inverting the bag prior to infusion. If needed, gently massage the bag to disperse cell clumps. Do not infuse the contents of an infusion bag if it is damaged or leaking, or otherwise appears to be compromised.
Infusion of AMTAGVI
- 10. Before infusion, the patient's health status should be reassessed and confirmed to be acceptable prior to AMTAGVI and IL-2 administration.
- 11. Confirm the patient's identity matches with the patient identifiers on the infusion bag.
- 12. Do NOT use a leukocyte depleting filter with AMTAGVI.
- 13. Prime the tubing with normal saline prior to infusion.
- 14. Initiate the infusion. Infuse the entire contents of each bag as soon as possible but within 3 hours of thawing.
- 15. Administer AMTAGVI at an infusion rate of approximately 1 mL per minute for the initial 5 minutes; thereafter 5 mL to 10 mL per minute.
- 16. Contents of all bags must be infused to complete a single dose. After the last bag is infused, rinse the tubing with normal saline at the same infusion rate to ensure all product is delivered.
AMTAGVI contains human cells. Follow universal and local biosafety guidelines applicable for the handling and disposal of AMTAGVI to avoid potential transmission of infectious diseases.
Administration of IL-2 (aldesleukin)
Beginning 3 to 24 hours after AMTAGVI infusion, administer intravenous IL-2 (aldesleukin) at 600,000 IU/kg every 8 to 12 hours for up to a maximum of 6 doses to support cell expansion in vivo. IL-2 (aldesleukin) should be administered in an inpatient setting under the supervision of a physician experienced in the use of anticancer agents.
-
3 DOSAGE FORMS AND STRENGTHS
AMTAGVI is a cell suspension for intravenous infusion.
A single dose of AMTAGVI contains 7.5 × 109 to 72 × 109 viable cells suspended in a cryopreservation medium. A single dose is split into 1 to 4 patient-specific infusion bag(s) (100 mL to 125 mL per bag) in individual protective metal cassettes [see How Supplied/Storage and Handling (16)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Treatment-Related Mortality
AMTAGVI is associated with treatment-related mortality. In the clinical trial, the treatment-related mortality rate was 7.5% (N=160), including 2 deaths during the lymphodepleting period, 6 deaths within 30 days, and 4 deaths 38 to 150 days following AMTAGVI administration. Adverse reactions associated with these deaths included severe infections (sepsis, pneumonia and encephalitis), internal organ hemorrhage (abdominal hemorrhage and intracranial hemorrhage), acute renal failure, acute respiratory failure, cardiac arrythmia, extensive ascites, liver injury, and bone marrow failure. Because clinical trials are conducted under widely varying conditions, treatment-related mortality rates observed in the clinical trials of a drug may not reflect the rates observed in practice.
5.2 Prolonged Severe Cytopenia
Patients treated with AMTAGVI may exhibit Grade 3 or higher cytopenia for weeks or longer. Based on adverse event reporting, Grade 3 or higher cytopenia or pancytopenia which did not resolve to less than or equal to Grade 2 or lasted beyond 30 days post AMTAGVI infusion occurred in 45.5% of melanoma patients who received AMTAGVI. Prolonged cytopenia included thrombocytopenia (30.1%), lymphopenia (19.9%), neutropenia (17.3%), leukopenia (14.7%), and pancytopenia (1.3%). Monitor blood counts after AMTAGVI infusion.
5.3 Internal Organ Hemorrhage
Patients treated with AMTAGVI may exhibit internal organ hemorrhage. Intraabdominal and intracranial hemorrhage can be life-threatening and have been associated with at least two deaths in patients who received AMTAGVI. Withhold or discontinue AMTAGVI infusion if internal organ hemorrhage is indicated, or patient is deemed ineligible for IL-2 (aldesleukin) infusion. Patients with persistent or repeated thrombocytopenia after receiving AMTAGVI should not use anticoagulants or must be under close monitoring if the patient must take anticoagulants.
5.4 Severe Infection
Severe, life-threatening, or fatal infections occurred in patients after AMTAGVI infusion. AMTAGVI treatment-related infections (any severity) occurred in 26.9% of patients with melanoma. Grade 3 or higher infections occurred in 13.5% of patients, including 10.9% of patients with infections of an unspecified pathogen and 3.8% of patients with infections of a specified pathogen.
Do not administer AMTAGVI to patients with clinically significant systemic infections. Monitor patients for signs and symptoms of infection before and after AMTAGVI infusion and treat appropriately. Administer prophylactic antimicrobials according to institutional guidelines.
Febrile neutropenia was observed in 46.8% of patients with melanoma after AMTAGVI Infusion. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
5.5 Cardiac Disorder
Patients treated with AMTAGVI may exhibit cardiac disorder. Grade 3 or higher cardiac disorders related to the AMTAGVI regimen occurred in 9.0% (14/156) of patients who received AMTAGVI including tachycardia, atrial fibrillation, arrhythmia, acute myocardial infarction, cardiac ventricular thrombosis, cardiomyopathy, QT-prolongation. Cardiac arrhythmia resulted in one death among melanoma patients who received AMTAGVI.
Monitor patients with signs and symptoms of cardiac disorder before and after AMTAGVI infusion. Withhold or discontinue AMTAGVI infusion, if severe cardiac disorder is indicated, or patient is deemed ineligible for IL-2 (aldesleukin) infusion.
5.6 Respiratory Failure
Patients treated with AMTAGVI may develop worsened respiratory function which has been associated with deaths. Monitor patients with signs and symptoms of respiratory failure before and after AMTAGVI infusion. Withhold or discontinue AMTAGVI infusion if severe acute respiratory failure is indicated, or patient is deemed ineligible for IL-2 (aldesleukin) infusion.
5.7 Acute Renal Failure
Patients treated with AMTAGVI may develop worsened renal function which has been associated with deaths. Monitor patients with signs and symptoms of acute renal failure before and after AMTAGVI infusion. Withhold or discontinue AMTAGVI if severe acute renal injury is indicated, or patient is deemed ineligible for IL-2 (aldesleukin) infusion.
5.8 Hypersensitivity Reactions
Allergic reactions including serious hypersensitivity (e.g., anaphylaxis) may occur with the infusion of AMTAGVI.
Acute infusion reactions (defined as occurring within 1 day of infusion) may occur and include fever, rigors or chills, tachycardia, rash, hypotension, dyspnea, cough, chest tightness, and wheezing. These events generally resolve on the same day of infusion. Patients should be monitored during and after infusion for signs and symptoms of a severe reaction, and treated promptly.
-
6 ADVERSE REACTIONS
The most common (incidence of greater than or equal to 20%) non-laboratory adverse reactions in order of decreasing frequency were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash, hypotension, alopecia, infection, hypoxia, and dyspnea.
The serious adverse reactions included:
- Treatment-Related Mortality [see Warnings and Precautions (5.1)]
- Prolonged Severe Cytopenia [see Warnings and Precautions (5.2)]
- Internal Organ Hemorrhage [see Warnings and Precautions (5.3)]
- Severe Infection [see Warnings and Precautions (5.4)]
- Cardiac Disorder [see Warnings and Precautions (5.5)]
- Respiratory Failure [see Warnings and Precautions (5.6)]
- Acute Renal Failure [see Warnings and Precautions (5.7)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety data described in this section reflect exposure to AMTAGVI within a regimen that included cyclophosphamide, fludarabine, and IL-2 (aldesleukin) in the global, multicenter, multicohort, open-label, single-arm clinical study in which 156 adult patients with unresectable or metastatic melanoma received a single infusion of AMTAGVI [see Clinical Studies (14)]. The median age of the study population was 56 years (range: 20 to 79 years); 53.8% were men. The performance status prior to tumor procurement was 68.6% with ECOG 0 and 31.4% with ECOG 1.
Table 1 summarizes the adverse reactions that occurred in at least 10% of patients treated with AMTAGVI and Table 2 describes the laboratory abnormalities of Grade 3 or 4 that occurred in at least 10% of patients.
Table 1: Adverse Reactions Observed in at Least 10% of Melanoma Patients Treated with AMTAGVI (N=156) Adverse Reaction Any Grade
n (%)Grade 3 or Higher
n (%)Adverse Reactions occurred from AMTAGVI infusion to 6 months (182 days) post infusion. - * Tachycardia includes tachycardia and sinus tachycardia, atrial fibrillation, supraventricular tachycardia.
- † Fatigue includes fatigue, asthenia, and malaise.
- ‡ Edema includes edema, face edema, generalized edema, localized edema, edema peripheral, peripheral swelling, edema genital, scrotal edema, brain edema, catheter site edema, conjunctival edema, eyelid edema, laryngeal edema, macular edema, periorbital edema, pulmonary edema, vasogenic cerebral edema, and lymphoedema.
- § Infection with unspecified pathogen includes cellulitis, conjunctivitis, cystitis, dermatitis infected, device related infection, diarrhea infectious, endocarditis, enterocolitis infectious, infection, meningitis, nasopharyngitis, neutropenic sepsis, pneumonia, pyuria, rash pustular, respiratory tract infection (RTI), rhinitis, sepsis, sinusitis, skin infection, urinary tract infection (UTI).
- ¶ Infection with mentioned pathogen includes bacteremia, candida infection, clostridium difficile colitis, cytomegalovirus infection or reactivation, Epstein-Barr virus infection, escherichia bacteremia, fungal skin infection, herpes simplex, herpes zoster, metapneumovirus infection, oral herpes, oral candidiasis, pneumonia klebsiella, respiratory syncytial virus infection, skin candida, tuberculosis.
- # Encephalopathy includes encephalopathy, automatism, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, hypersomnia, lethargy, leukoencephalopathy, memory impairment, mental status changes, paranoia, somnolence, and stupor.
- Þ Acute kidney injury includes acute kidney injury, anuria, azotemia, renal failure, renal tubular dysfunction, renal tubular necrosis, oliguria, and blood creatinine increased.
- ß Hypoxia includes hypoxia and oxygen saturation decreased.
- à Dyspnea includes dyspnea, acute respiratory failure, orthopnea, respiratory distress, respiratory failure, and dyspnea exertional
- è Rash includes rash, rash generalized, rash maculo-papular, rash papular, rash pruritic, rash erythematous, and rash macular.
- ð Hypotension includes hypotension, blood pressure decreased, blood pressure systolic decreased, blood pressure diastolic decreased, and orthostatic hypotension.
- ø Hypertension includes hypertension, blood pressure increased, blood pressure systolic increased, and blood pressure diastolic increased.
Blood and lymphatic system disorders Febrile neutropenia 73 (46.8) 73 (46.8) Cardiac disorders Tachycardia* 74 (47.4) 12 (7.7) Gastrointestinal disorders Diarrhea 73 (46.8) 3 (1.9) Vomiting 68 (43.6) 2 (1.3) Nausea 107 (68.6) 4 (2.6) General disorders and administration site conditions Chills 118 (75.6) 8 (5.1) Pyrexia 95 (60.9) 16 (10.3) Fatigue† 87 (55.8) 8 (5.1) Edema‡ 66 (42.3) 8 (5.1) Investigations Weight increased 30 (19.2) 2 (1.3) Infections and Infestations 42 (26.9) 21 (13.5) Infection with pathogen unspecified§ 30 (19.2) 17 (10.9) Infection with pathogen specified¶ 19 (12.2) 6 (3.8) Metabolism and nutrition disorders Decreased appetite 48 (30.8) 2 (1.3) Nervous system disorders Headache 33 (21.2) 1 (0.6) Encephalopathy# 27 (17.3) 9 (5.8) Renal and urinary disorders Acute kidney injuryÞ 31 (19.9) 11 (7.1) Hematuria 22 (14.1) 2 (1.3) Respiratory, thoracic and mediastinal disorders Hypoxiaß 37 (23.7) 19 (12.2) Dyspneaà 34 (21.8) 13 (8.3) Skin and subcutaneous tissue disorders Rashè 58 (37.2) 15 (9.6) Alopecia 48 (30.8) 0 (0) Pruritus 21 (13.5) 0 (0) Vascular disorders Hypotensionð 58 (37.2) 17 (10.9) Capillary leak syndrome 21 (13.5) 7 (4.5) Hypertensionø 21 (13.5) 11 (7.1) Adverse reactions that occurred in less than 10% of patients treated with AMTAGVI included the following:
- Eye disorders: Uveitis (4.5%). Other eye disorders included Grade 1 or 2 retinal detachment, vision blurred, visual impairment, periorbital edema, visual acuity reduced, and retinal hemorrhage.
- Immune system disorders: Infusion related reaction (6.4%), anaphylactic reaction (1.3%), and cytokine release syndrome (3.2%).
- Skin and subcutaneous tissue disorders: Vitiligo (7.1%).
Table 2: Grade 3 or 4 Laboratory Abnormalities Occurring in at Least 10% of Melanoma Patients Following Treatment with AMTAGVI (N=156) Laboratory Abnormality Grades 3 or 4 (%) Frequency of Grade 3 or 4 laboratory abnormalities from AMTAGVI infusion to 6 months (182 days) post infusion. Thrombocytopenia 122 (78.2) Neutropenia 108 (69.2) Anemia 91 (58.3) Leukopenia 73 (46.8) Lymphopenia 66 (42.3) Hypophosphatemia 40 (25.6) Serious Adverse Reactions
Serious adverse reactions leading to death included acute respiratory failure (n=1), renal failure (n=2), cardiac arrhythmia (n=1), severe infections (n=4) including sepsis and septic shock, pneumonia, and encephalitis, internal organ hemorrhage (n=2), ascites and liver injury (n=1) and bone marrow failure (n=1).
Deaths
Among 160 patients with unresectable, or metastatic melanoma who initiated the AMTAGVI regimen, there were 12 deaths (7.5%), including 2 deaths during the lymphodepleting period, 6 deaths within 30 days following AMTAGVI administration, and additional 4 deaths 38 to 150 days following AMTAGVI administration. Adverse reactions associated with these deaths included severe infections (sepsis, pneumonia and encephalitis), internal organ hemorrhage (abdominal hemorrhage and intracranial hemorrhage), acute renal failure, acute respiratory failure, cardiac arrythmia, extensive ascites and liver injury and bone marrow failure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with AMTAGVI use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with AMTAGVI. Therefore, AMTAGVI is not recommended for women who are pregnant, and pregnancy after AMTAGVI administration should be discussed with the treating physician. Report pregnancies to Iovance Biotherapeutics, Inc. at 1-833-400-IOVA.
In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of AMTAGVI in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AMTAGVI and any potential adverse effects on the breastfed infant from AMTAGVI or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
-
11 DESCRIPTION
AMTAGVI (lifileucel) is a tumor-derived autologous T cell immunotherapy comprised of a suspension of tumor-derived T cells for intravenous infusion. AMTAGVI is manufactured from resected patient tumor tissue prosected from one or more tumor lesions. Immune cells derived from a patient's tumor(s) are expanded in cell culture, washed, formulated as a cell suspension, and cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in 1 to 4 patient-specific infusion bag(s) in individual protective metal cassettes. The product is thawed prior to administration back into the same patient [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
AMTAGVI is composed primarily of T cells of the CD4+T and CD8+T cell lineages. AMTAGVI may also contain monocytes and other lymphocytes, including B cells and NK cells. AMTAGVI may contain viable melanoma tumor cells from the original tumor tissue used to manufacture the product.
The formulation contains 48% PlasmaLyte A, 50% CryoStor CS10 (resulting in final concentration of 5% dimethyl sulfoxide (DMSO)), 2% of 25% human serum albumin (resulting in a final concentration of 0.5% albumin), and 300 IU/mL IL-2 (aldesleukin).
A single dose of AMTAGVI is provided in 1 to 4 infusion bag(s) containing 100 mL to 125 mL of viable cells per bag in individual protective cassettes.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Pharmacodynamic activity was evaluated by measuring longitudinal changes of cytokines and chemokines (IL-15, IL-6, IL-7, IL-9, IL-10, IL-12(p40), CCL2, CXCL10, IFN-γ, and TNF-α) using plasma samples collected at baseline and post-infusion of AMTAGVI up to Month 3. The mean level of IL-15 and CXCL10 peaked following lymphodepletion and administration of AMTAGVI at Day 1-4, decreased over time, and returned to baseline levels within 1-3 months. The mean IFN-γ level was below baseline post lymphodepletion and AMTAGVI infusion at Day 1-4 and returned to baseline by Day 14. Other cytokines and chemokines listed above did not show any noticeable changes. No difference was observed in the cytokines and chemokines level between responding and non-responding patients.
12.3 Pharmacokinetics
The proportion of unique TCR clonotypes from the AMTAGVI lots contributing to the peripheral blood TCR repertoire among infused patients was analyzed using a semi-quantitative polymerase chain reaction followed by next generation sequencing. The proportion of TCR clones that are composed of clonotypes identified in the product increases from a mean of 16% (n=125) at pre-infusion to 83% at Day 4 after AMTAGVI infusion. The TCR clones declined to 51% at Day 14 (n=51) and remain 37% to 41% from Day 42 (n=120) to month 12 (n=37) post-infusion of AMTAGVI. No significant correlation was found between AMTAGVI persistence and efficacy.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of a single treatment with AMTAGVI was evaluated in a global, multicenter, multicohort, open-label, single-arm clinical study. This study enrolled patients with unresectable or metastatic melanoma who had previously been treated with at least one systemic therapy, including a PD-1 blocking antibody, and if BRAF V600 mutation-positive, a BRAF inhibitor or BRAF inhibitor with MEK inhibitor. This study excluded patients with uncontrolled brain metastases, organ allograft or prior cell transfer, melanoma of uveal or ocular origin, systemic steroid therapy for any reason, Grade 2 or higher hemorrhage within 14 days prior to study enrollment (tumor resection), left ventricular ejection fraction (LVEF) less than 45% or New York Heart Association (NYHA) functional classification greater than Class 1, and patients with forced expiratory volume in one second (FEV1) of less than or equal to 60%.
There were 111 patients who underwent tumor resection of whom 22 (19.8%) patients did not receive AMTAGVI due to the following reasons: inability to manufacture AMTAGVI (n=6), disease related death (n=3), meeting exclusion criteria (n=5), disease progression (n=3), starting new anti-cancer therapy or consent withdrawal (n=3), or adverse events from lymphodepletion including one death (n=2). Among 89 patients who received AMTAGVI, two (2) patients were excluded because the product did not meet specification and five (5) patients were excluded due to product comparability.
The primary efficacy analysis set included 82 patients who received AMTAGVI. Among these, nine (9) patients received AMTAGVI at a dose less than 7.5 × 109 viable cells and did not achieve an objective response. The recommended AMTAGVI dosing range was set at 7.5 × 109 to 72 × 109 viable cells (73 patients received this dosing range).
Median time from tumor tissue procurement to the end of the manufacturing process was 23 days and to infusion was 34 days. Lesion origin of AMTAGVI products included skin, lymph nodes, liver, lung, peritoneal, musculoskeletal, breast, and other anatomic sites including chest wall, abdominal wall, adrenal gland, abdominal-peritoneal, paraesophageal, axillary, thigh, back, supraclavicular, and soft tissue.
All 73 (100%) patients received prior anti-PD-(L)1 therapy, 63 (86.3%) received prior anti-CTLA-4 therapy, 42 (57.5%) received anti-PD1/anti-CTLA-4 combination therapy and 20 (27.4%) received a BRAF inhibitor or combination therapy with BRAF and MEK inhibitors. The median age was 58 years (min, max: 25, 74 years), with 26.0% age 65 or older, 52.1% were male, 94.5% were white, 2.7% were black, and 1.4% were Asian. Patients received a median of 3 prior lines of therapy and a median of 2 prior lines of anti-PD(L)1 containing therapies. Disease characteristics were: BRAF V600 mutation-positive: 27.4%; PD-L1 TPS greater than or equal 5%: 23.3%; elevated LDH: 63.0%; brain and/or liver metastases: 54.8%. The median target lesion sum of diameters was 108.7 mm (min, max: 15.7, 552.9). The performance status prior to tumor procurement was ECOG 0 (71.2%) and ECOG 1 (28.8%).
AMTAGVI was administered following a lymphodepleting regimen consisting of cyclophosphamide 60 mg/kg daily with mesna for 2 days followed by fludarabine 25 mg/m2 daily for 5 days. Three (3) to 24 hours after infusion, patients received IL-2 (aldesleukin) at 600,000 IU/kg every 8 to 12 hours for up to 6 doses in order to support cell expansion in vivo. The median administered AMTAGVI dose was 21.1× 109 viable cells. The median number of administered IL-2 (aldesleukin) doses was 6.
Efficacy was established on the basis of objective response rate (ORR) and duration of response (DoR) (see Table 3). The median time to initial response to AMTAGVI was 1.5 months (min, max: 1.3, 4.2).
Table 3: Efficacy Results Among Patients Who Received AMTAGVI Dose Range of 7.5 × 109 to 72 × 109 Viable Cells Endpoint* Efficacy Set (N=73) CI, confidence interval; DoR, duration of response; NR, not reached; ORR, objective response rate. - * Per RECIST v1.1 assessed by Independent Review Committee (IRC).
- † Number of responders was N=23.
- ‡ Kaplan-Meier estimate of median potential follow-up for DoR was 18.6 months.
- § Kaplan-Meier estimate in months among all responders. DoR measured from the date of confirmed initial objective response to the date of progression or death from any cause.
- ¶ + sign indicates a censored value
- # Observed proportion of patients with duration of response beyond landmark time
Objective Response Rate ORR, % (95% CI) 31.5 (21.1, 43.4) Complete response rate, n (%) 3 (4.1) Partial response rate, n (%) 20 (27.4) Duration of Response†, ‡ Median DoR in months (95% CI)§ NR (4.1, NR) Range¶ (1.4+, 26.3+) Patients with DoR ≥ 6 months#, n (%) 13 (56.5) Patients with DoR ≥ 9 months#, n (%) 11 (47.8) Patients with DoR ≥ 12 months#, n (%) 10 (43.5) Supporting pooled efficacy analysis included 189 patients who underwent tumor resection of whom 33 did not receive AMTAGVI due to the following reasons: inability to manufacture AMTAGVI (n=8), disease related death within 40 days after tumor harvest (n=5), meeting exclusion criteria (n=6), disease progression (n=6), starting new anti-cancer therapy or consent withdrawal (n=4), or adverse events from lymphodepletion including two deaths (n=4). Among the 156 patients who received AMTAGVI, 2 patients who received AMTAGVI that did not meet the product specification and 1 patient who received AMTAGVI below the protocol specified dosing range due to anaphylactic reaction were excluded.
The supporting pooled efficacy set included 153 patients. The median administered AMTAGVI dose was 21.1× 109 viable cells and the median number of administered IL-2 (aldesleukin) doses was 6. The ORR was 31.4% (95% CI: 24.1%, 39.4%) with a CR of 5.2% (n=8) and PR of 26.1% (n=40). The median time to initial response to AMTAGVI was 1.5 months (min, max: 1.3, 4.2). The median DoR was not reached (range: 1.4+, 45.0+). Among responders, 62.5%, 56.3% and 54.2% maintained durable responses at 6, 9 and 12 months, respectively, following the initial response.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AMTAGVI is supplied in 1 to 4 infusion bag(s) (NDC: 73776-001-11), with each bag containing approximately 100 mL to 125 mL of frozen suspension of tumor-derived T cells in 5% DMSO, 0.5% albumin (human), and 300 IU/mL IL-2 (aldesleukin). Each bag is contained within a protective metal cassette (NDC: 73776-001-12). AMTAGVI is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen cryoshipper.
Product and patient-specific labels are located on both the product infusion bag(s) and the protective shipping cassette(s), which are inside the liquid nitrogen cryoshipper.
- Match the identity of the patient with the patient identifiers on the cassette(s) and infusion bag(s) upon receipt.
- Confirm the number of AMTAGVI cassette(s) and infusion bag(s) matches the total number of cassette(s) and infusion bag(s) on the shipment packing slip.
- Store AMTAGVI frozen in the vapor phase of liquid nitrogen (less than or equal to minus 150°C).
- Thaw AMTAGVI immediately prior to infusion [see Dosage and Administration (2.2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Prior to infusion, advise patients of the following risks:
Hypersensitivity Reactions:
Signs and symptoms associated with hypersensitivity reactions including fever, rigors or chills, tachycardia, rash, hypotension, dyspnea, cough, chest tightness, and wheezing [see Warnings and Precautions (5.8)].
Prolonged Severe Cytopenia:
Signs or symptoms associated with bone marrow suppression (i.e., neutropenia, thrombocytopenia and anemia) for more than several weeks following lymphodepleting chemotherapy and AMTAGVI [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
Severe Infection:
Signs and symptoms associated with severe infections. Advise patients that AMTAGVI treatment regimen will not be initiated if they have an ongoing uncontrolled infection. Anti-microbial prophylaxis and treatment of infection will be administered [see Warnings and Precautions (5.4), Adverse Reactions (6.1)].
Internal Organ Hemorrhage:
Signs or symptoms of internal organ hemorrhage following AMTAGVI treatment regimen. Advise patients to seek immediate medical attention should signs or symptoms of internal organ hemorrhage occur at any time [see Warnings and Precautions (5.3)].
Cardiopulmonary and Renal Impairment:
Signs or symptoms of cardiopulmonary or renal injuries following AMTAGVI treatment regimen. Advise patients to seek immediate medical attention should signs or symptoms of cardiopulmonary, or renal injury occur at any time [see Warnings and Precautions (5.5, 5.6, 5.7)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Advanced Therapies, LLC.
4000 South 26th Street
Philadelphia, PA 19112OR
Iovance Biotherapeutics Manufacturing LLC
300 Rouse Boulevard
Philadelphia, PA 19112For specific manufacturing site used to manufacture the received AMTAGVI batch, see bag and cassette labels.
Marketed by:
Iovance Biotherapeutics Manufacturing LLC
300 Rouse Boulevard
Philadelphia, PA 19112
U.S. license number: 2298© 2024 Iovance Biotherapeutics, Inc.
-
Patient InformationAMTAGVI (pronounced am-tag-vee)(lifileucel)
What is the most important information I should know about AMTAGVI?
- You will likely be in a hospital prior to and after receiving AMTAGVI.
What is AMTAGVI?
AMTAGVI is a type of medicine called a 'Tumor-derived autologous T cell immunotherapy.' The active substance in the medicine is lifileucel, which is comprised of T cells derived from tissue excised from your tumor. Tumor-derived T cells are cells produced by the body's immune system that may recognize and kill cancer (tumor) cells.
AMTAGVI is used to treat adult patients with a type of skin cancer that cannot be removed surgically or has spread to other parts of the body called unresectable or metastatic melanoma. AMTAGVI is used when your melanoma has not responded or stopped responding to a PD-1 blocking drug either by itself or in a combination, and if your cancer is BRAF mutation positive, a BRAF inhibitor drug with or without a MEK inhibitor drug that also has stopped working.
Before taking AMTAGVI, tell your healthcare provider about all of your medical conditions, including if you:
- Have any lung, heart, liver or kidney problems
- Have low blood pressure
- Have a recent or active infection or other inflammatory conditions including cytomegalovirus (CMV) infection, hepatitis B or C or human immunodeficiency virus (HIV) infection
- Are pregnant, think you may be pregnant, or plan to become pregnant
- Are breastfeeding
- Notice the symptoms of your cancer are getting worse
- Have had a vaccination in the past 28 days or plan to have one in the next few months
- Have been taking blood thinner
Tell your healthcare provider about all the medications you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive AMTAGVI?
- AMTAGVI is made from your surgically removed tumor. Tumor derived T cells are grown in a manufacturing center at the end of which they number in the billions of cells.
- Your tumor tissue is sent to a manufacturing center to make AMTAGVI. It takes about 34 days from the time your tumor tissue is received at the manufacturing center until AMTAGVI is available to be shipped back to your healthcare provider, but the time may vary. Your AMTAGVI will be provided in 1 to 4 patient-specific infusion bag(s) containing 100 mL to 125 mL of viable (alive) cells per bag.
- After your AMTAGVI arrives at your treating institution, your healthcare provider will give you lymphodepleting chemotherapy to prepare your body.
- Approximately 30 to 60 minutes before you are given AMTAGVI, you may be given other medicines. These may include:
- Medicines for an allergic reaction (anti-histamines)
- Medicines for fever (such as acetaminophen)
- Your AMTAGVI will be provided in 1 to 4 infusion bag(s) containing 100 mL to 125 mL of viable cells per bag. When your body is ready for AMTAGVI infusion, your healthcare provider will give AMTAGVI to you by intravenous infusion. This usually takes less than one and a half hours.
After getting AMTAGVI
Beginning 3 to 24 hours after AMTAGVI is given, you may be given up to 6 doses of IL-2 (aldesleukin) every 8 to 12 hours via intravenous infusion. Your doctor may discontinue IL-2 (aldesleukin) infusion any time if you present severe side effects.
You will have to stay in the hospital until you have completed the IL-2 (aldesleukin) treatment and you have recovered from any serious side effects associated with the AMTAGVI treatment.
You should plan to stay within 2 hours of the location where you received your treatment for several weeks after getting AMTAGVI. Your healthcare provider will check to see if your treatment is working and help you with any side effects that occur.
What are the possible side effects of AMTAGVI?
The most common side effects of the AMTAGVI treatment include:
- Chills
- Fever (100.4°F (38°C) or higher)
- Low white blood cell count (may increase risk of infections)
- Fatigue
- Low red blood cell count (may cause you to feel tired or weak)
- Fast or irregular heartbeat
- Rash
- Low blood pressure
- Diarrhea
These are not all the possible side effects of the AMTAGVI treatment. If you would like more information about AMTAGVI, talk with your healthcare provider. You can ask your healthcare provider for information about AMTAGVI that is written for health professionals. You may report side effects to FDA at 1-800-FDA-1088.
Marketed by:
Iovance Biotherapeutics Manufacturing LLC
Philadelphia, PA 19112 -
PRINCIPAL DISPLAY PANEL - 125 mL Bag Cartridge Label - Iovance
NDC: 73776-001-12
Human T-cells Suspension
for Intravenous Infusion
For autologous and
intravenous use onlylifileucel
AMTAGVI®Rx only
See package insert for full dosage and
administration instructionsDose: 1 to 4 bags, infuse within 3 hours
Do not use leukocyte depleting filter
Target total volume: 100 mL to 125 mL per bag
Contents: 7.5x109 to 72x109 viable cells cryopreserved
in 5% DMSO, 0.5% albumin (human), and
300 IU/mL IL-2 (aldesleukin)May contain trace amounts of gentamicin, streptomycin,
and amphotericin BNo preservative, No U.S. Standard of Potency
Not evaluated for infectious substancesStore at ≤ -150°C in vapor phase liquid nitrogen
DO NOT IRRADIATE
Thaw immediately before use, hold at 18-25°C
DO NOT REFREEZE
Mfd by:Iovance Biotherapeutics Manufacturing LLC
300 Rouse Blvd., Philadelphia, PA 19112IOVANCE
BIOTHERAPEUTICSTumor Collection Date: 2024-02-13
Intended Recipient: Last Name, First Name
Recipient ID: N/A
Date of Birth: 1996-02-08
COI: 202402071A
DIN: 1234567
Lot: 012345678
Expiry: 2024-04-17
Product Code: S35431ISBT 128
Bag Number: 1 of 4
U.S. License# 2298 Amtagvi.com
1-833-400-IOVA (1-833-400-4682)
©2024 Iovance Biotherapeutics, Inc.
-
PRINCIPAL DISPLAY PANEL - 125 mL Bag Cartridge Label - Advanced Therapies
NDC: 73776-001-12
Human T-cells Suspension
for Intravenous Infusion
For autologous and
intravenous use onlylifileucel
AMTAGVI®Rx only
See package insert for full dosage and
administration instructionsDose: 1 to 4 bags, infuse within 3 hours
Do not use leukocyte depleting filter
Target total volume: 100 mL to 125 mL per bag
Contents: 7.5x109 to 72x109 viable cells cryopreserved
in 5% DMSO, 0.5% albumin (human), and
300 IU/mL IL-2 (aldesleukin)May contain trace amounts of gentamicin, streptomycin,
and amphotericin BNo preservative, No U.S. Standard of Potency
Not evaluated for infectious substancesStore at ≤ -150°C in vapor phase liquid nitrogen
DO NOT IRRADIATE
Thaw immediately before use, hold at 18-25°C
DO NOT REFREEZE
Mfd by: Advanced Therapies, LLC.
4000 South 26th Street, Philadelphia, PA 19112IOVANCE
BIOTHERAPEUTICSTumor Collection Date: 2024-02-13
Intended Recipient: Last Name, First Name
Recipient ID: N/A
Date of Birth: 1996-02-08
COI: 202402071A
DIN: 1234567
Lot: 012345678
Expiry: 2024-04-17
Product Code: S35431ISBT 128
Bag Number: 1 of 4
U.S. License# 2298 Amtagvi.com
1-833-400-IOVA (1-833-400-4682)
©2024 Iovance Biotherapeutics, Inc.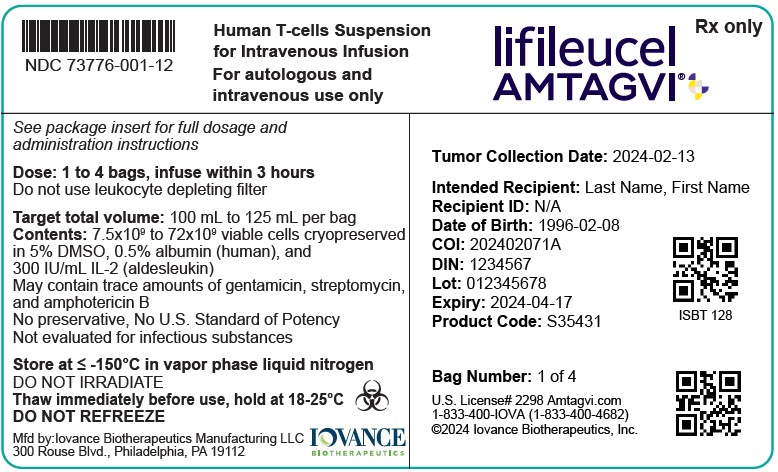
-
INGREDIENTS AND APPEARANCE
AMTAGVI
lifileucel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC: 73776-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lifileucel (UNII: R0835E18NH) (lifileucel - UNII:R0835E18NH) lifileucel 72000000000 in 500 mL Inactive Ingredients Ingredient Name Strength Dimethyl Sulfoxide (UNII: YOW8V9698H) Aldesleukin (UNII: M89N0Q7EQR) Plasmalyte A (UNII: MZD2VV6EW6) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73776-001-12 1 in 1 CARTRIDGE 1 NDC: 73776-001-11 125 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125773 02/16/2024 Labeler - Iovance Biotherapeutics Inc. (962508433) Establishment Name Address ID/FEI Business Operations Iovance Biotherpeutics Manufacturing, LLC 118423955 MANUFACTURE(73776-001) Establishment Name Address ID/FEI Business Operations Advanced Therapies, LLC. 080416125 MANUFACTURE(73776-001)
Trademark Results [AMTAGVI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AMTAGVI 97930602 not registered Live/Pending |
Iovance Biotherapeutics, Inc. 2023-05-10 |
 AMTAGVI 88918260 not registered Live/Pending |
Iovance Biotherapeutics, Inc. 2020-05-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.