ASPIRIN by MWI (Vet One) / Sparhawk Laboratories, Inc. Aspirin Powder
ASPIRIN by
Drug Labeling and Warnings
ASPIRIN by is a Animal medication manufactured, distributed, or labeled by MWI (Vet One), Sparhawk Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ASPIRIN- acetylsalicylic acid powder
MWI (Vet One)
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
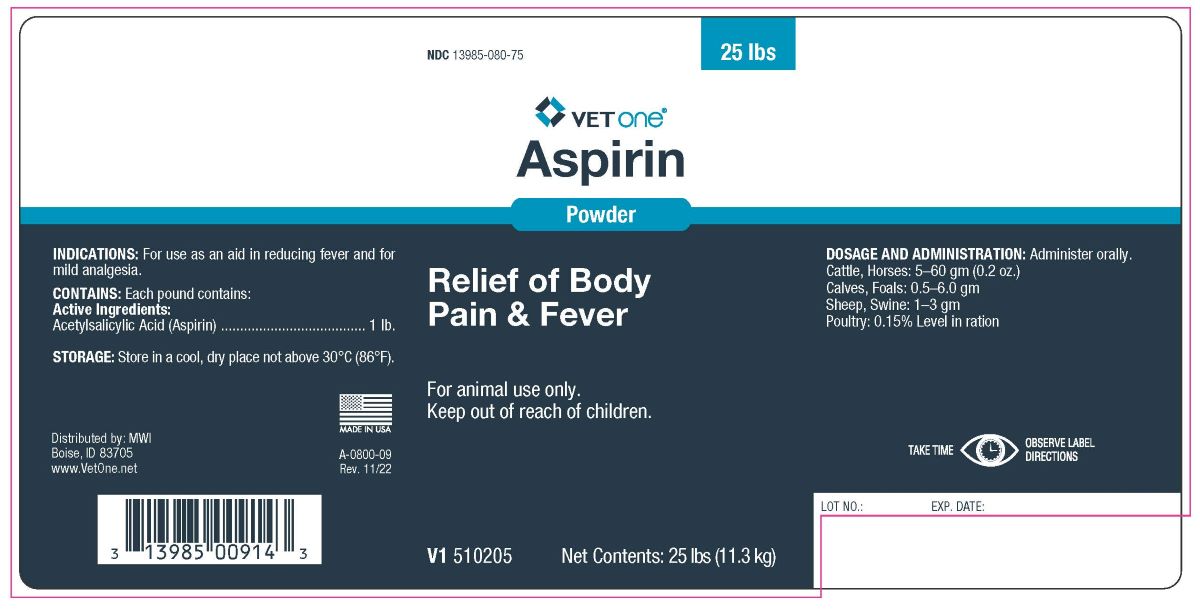
Aspirin Powder
For animal use only.
Keep out of reach of children.
INDICATIONS
For use as an aid in reducing fever and for
mild analgesia.
DOSAGE AND ADMINISTRATION
Administer orally.
Cattle, Horses: 5–60 gm (0.2 oz.)
Calves, Foals: 0.5–6.0 gm
Sheep, Swine: 1–3 gm
Poultry: 0.15% Level in ration
| ASPIRIN
acetylsalicylic acid powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - MWI (Vet One) (019926120) |
| Registrant - Sparhawk Laboratories, Inc. (147979082) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sparhawk Laboratories, Inc. | 147979082 | analysis, manufacture, api manufacture | |
Revised: 12/2025
Document Id: fe443518-e492-4963-a8c7-2a1f07e89a57
Set id: 91dc74f3-b5b8-4851-a011-28852c91a3b2
Version: 3
Effective Time: 20251222
Trademark Results [ASPIRIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ASPIRIN 76481358 2788617 Dead/Cancelled |
Simon Carter Accessories, Ltd. 2003-01-10 |
 ASPIRIN 75209895 not registered Dead/Abandoned |
Bayer Aktiengesellschaft 1996-12-09 |
 ASPIRIN 73234351 1171777 Dead/Cancelled |
McIntyre; William A. 1979-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.