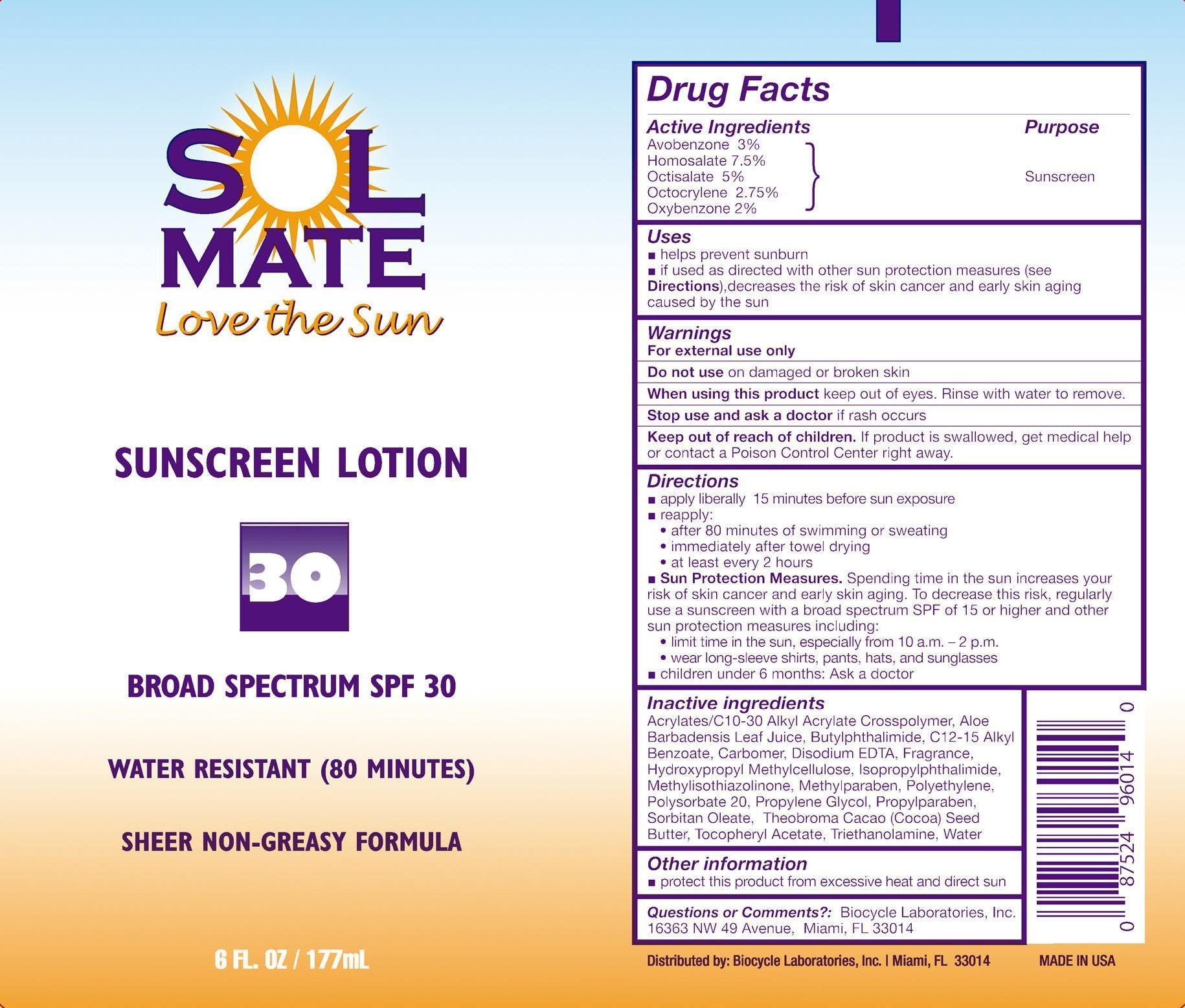
SOLMATE BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone lotion
SolMate by
Drug Labeling and Warnings
SolMate by is a Otc medication manufactured, distributed, or labeled by Prime Enterprises, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
-
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
-
- limit time in the sun especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Butylphthalimide, C12-15 Alkyl Benzoate, Carbomer, Disodium EDTA, Fragrance, Hydroxypropyl Methylcellulose, Isopropylphthalimide, Methylisothiazolinone, Methylparaben, Polyethylene, Polysorbate 20, Propylene Glycol, Propylparaben, Sorbitan Oleate, Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Triethanolamine, Water
- Other information
- QUESTIONS
- PRINCIPAL DISPLAY PANEL - 177 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
SOLMATE BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58443-0110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27.5 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength (C10-C30)ALKYL METHACRYLATE ESTER (UNII: XH2FQZ38D8) ALOE VERA LEAF (UNII: ZY81Z83H0X) N-BUTYLPHTHALIMIDE (UNII: 5TH1DKT35E) ISOPROPYLPHTHALIMIDE (UNII: 1J1MM83329) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) EDETATE DISODIUM (UNII: 7FLD91C86K) HYPROMELLOSES (UNII: 3NXW29V3WO) METHYLPARABEN (UNII: A2I8C7HI9T) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) COCOA BUTTER (UNII: 512OYT1CRR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58443-0110-4 177 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/19/2013 Labeler - Prime Enterprises, Inc. (101946028) Registrant - Prime Enterprises, Inc. (101946028) Establishment Name Address ID/FEI Business Operations Prime Enterprises, Inc. 101946028 label(58443-0110) , pack(58443-0110) , manufacture(58443-0110) , analysis(58443-0110)
Trademark Results [SolMate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SOLMATE 98567831 not registered Live/Pending |
Kraus Haus Designs, LLC 2024-05-24 |
 SOLMATE 98563992 not registered Live/Pending |
Bean's Best, LLC 2024-05-22 |
 SOLMATE 85578863 not registered Dead/Abandoned |
1 OAK SEATTLE, LLC 2012-03-23 |
 SOLMATE 85196023 not registered Dead/Abandoned |
Tal Adam Benzion 2010-12-12 |
 SOLMATE 85152563 4684918 Live/Registered |
BIOCYCLE LABORATORIES, INC. 2010-10-14 |
 SOLMATE 77236272 3473470 Dead/Cancelled |
BISHOP, LYNN 2007-07-23 |
 SOLMATE 74652842 2375551 Dead/Cancelled |
CALIFORNIA SUNCARE, INC. 1995-03-29 |
 SOLMATE 74151148 1680188 Dead/Cancelled |
RYBAK, FRANKLYN M. 1991-03-21 |
 SOLMATE 74077025 not registered Dead/Abandoned |
Excelsior, Inc. 1990-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.