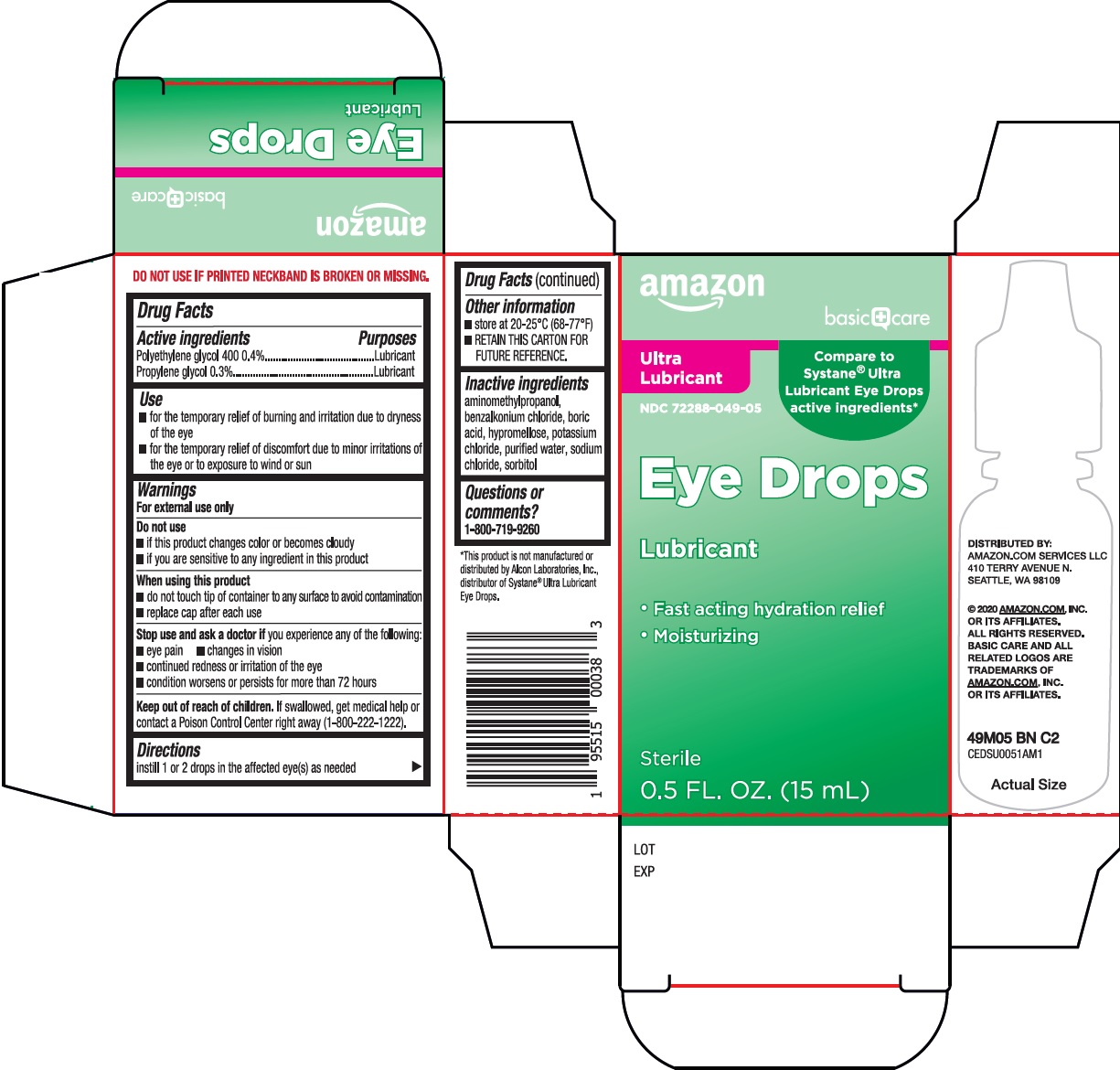
Amazon Eye Drops Drug Facts
Basic Care Eye by
Drug Labeling and Warnings
Basic Care Eye by is a Otc medication manufactured, distributed, or labeled by Amazon.com Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BASIC CARE EYE- polyethylene glycol, propylene glycol solution/ drops
Amazon.com Services LLC
----------
Amazon Eye Drops Drug Facts
Use
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
| BASIC CARE EYE
polyethylene glycol, propylene glycol solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amazon.com Services LLC (128990418) |
Revised: 11/2024
Document Id: d595b45f-bd0d-4fd4-929e-4edca8e1fe62
Set id: 91f046ce-5ed1-48bb-847e-3223da985c90
Version: 4
Effective Time: 20241113