These highlights do not include all the information needed to use CEFEPIME FOR INJECTION safely and effectively. See full prescribing information for CEFEPIME FOR INJECTION. CEFEPIME for injection, for intravenous or intramuscular use Initial U.S. Approval: 1996
CEFEPIME HYDROCHLORIDE by
Drug Labeling and Warnings
CEFEPIME HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Xellia Pharmaceuticals USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CEFEPIME HYDROCHLORIDE- cefepime hydrochloride injection, powder, for solution
Xellia Pharmaceuticals USA LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CEFEPIME FOR INJECTION safely and effectively. See full prescribing information for CEFEPIME FOR INJECTION.
CEFEPIME for injection, for intravenous or intramuscular use Initial U.S. Approval: 1996 INDICATIONS AND USAGECefepime for Injection is a cephalosporin antibacterial indicated for the treatment of the following infections caused by susceptible strains of the designated microorganisms:
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefepime for Injection and other antibacterial drugs, Cefepime for Injection should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. (1.6) DOSAGE AND ADMINISTRATION
Pediatric Patients (2 months to 16 years) Recommended dosage in pediatric with CrCL greater than 60 mL/min. (2.2) DOSAGE FORMS AND STRENGTHSCefepime for Injection is a sterile powder of cefepime in vials for reconstitution, available in the following strengths:
CONTRAINDICATIONSPatients with known immediate hypersensitivity reactions to cefepime or other cephalosporins, penicillins or other beta-lactam antibacterial drugs. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Xellia Pharmaceuticals USA, LLC at 1-833-295-6953, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 2/2022 |
|||||||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Pneumonia
Cefepime for Injection is indicated in the treatment of pneumonia (moderate to severe) caused by susceptible strains of Streptococcus pneumoniae, including cases associated with concurrent bacteremia, Pseudomonas aeruginosa, Klebsiella pneumoniae, or Enterobacter species.
1.2 Empiric Therapy for Febrile Neutropenic Patients
Cefepime for Injection as monotherapy is indicated for empiric treatment of febrile neutropenic patients. In patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia), antimicrobial monotherapy may not be appropriate. Insufficient data exist to support the efficacy of cefepime monotherapy in such patients [see Clinical Studies (14.1)].
1.3 Uncomplicated and Complicated Urinary Tract Infections (including pyelonephritis)
Cefepime for Injection is indicated in the treatment of uncomplicated and complicated urinary tract infections (including pyelonephritis) caused by susceptible isolates of Escherichia coli or Klebsiella pneumoniae, when the infection is severe, or caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis, when the infection is mild to moderate, including cases associated with concurrent bacteremia with these bacteria.
1.4 Uncomplicated Skin and Skin Structure Infections
Cefepime for Injection is indicated in the treatment of uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible isolates only) or Streptococcus pyogenes.
1.5 Complicated Intra-abdominal Infections (used in combination with metronidazole)
Cefepime for Injection is indicated in the treatment of complicated intra-abdominal infections (used in combination with metronidazole) in adults caused by susceptible isolates of Escherichia coli, viridans group streptococci, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter species, or Bacteroides fragilis [see Clinical Studies (14.2)].
1.6 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefepime for Injection and other antibacterial drugs, Cefepime for Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Adults
The recommended adult dosages and routes of administration are outlined in Table 1 below for patients with creatinine clearance greater than 60 mL/min. Administer cefepime for injection intravenously over approximately 30 minutes.
| Site and Type of Infection | Dose | Frequency | Duration (days) |
|---|---|---|---|
| Adults | Intravenous (IV)/Intramuscular (IM) | ||
|
|
|||
| Moderate to Severe Pneumonia* | 1 to 2 g IV | Every 8 to 12 hours | 10 |
| Empiric therapy for febrile neutropenic patients | 2 g IV | Every 8 hours | 7† |
| Mild to Moderate Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis | 0.5 to 1 g IV/IM‡ | Every 12 hours | 7 to 10 |
| Severe Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis | 2 g IV | Every 12 hours | 10 |
| Moderate to Severe Uncomplicated Skin and Skin Structure Infections | 2 g IV | Every 12 hours | 10 |
| Complicated Intra-abdominal Infections* (used in combination with metronidazole) | 2 g IV | Every 8 to 12 hours | 7 to 10 |
2.2 Pediatric Patients (2 months up to 16 years)
The maximum dose for pediatric patients should not exceed the recommended adult dose.
The usual recommended dosage in pediatric patients up to 40 kg in weight for durations as given above for adults is:
- 50 mg per kg per dose, administered every 12 hours for uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and pneumonia (see below).
- For moderate to severe pneumonia due to P. aeruginosa give 50 mg per kg per dose, every 8 hours.
- 50 mg per kg per dose, every 8 hours for febrile neutropenic patients.
2.3 Dosage Adjustment in Patients with Renal Impairment
Adult Patients
Adjust the dose of cefepime for injection in patients with creatinine clearance less than or equal to 60 mL/min to compensate for the slower rate of renal elimination. In these patients, the recommended initial dose of cefepime for injection should be the same as in patients with CrCL greater than 60 mL/min except in patients undergoing hemodialysis. The recommended doses of cefepime for injection in patients with renal impairment are presented in Table 2.
When only serum creatinine is available, the following formula (Cockcroft and Gault equation)1 may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function:
| Males: Creatinine Clearance (mL/min) = | Weight (kg) × (140 – age) |
| 72 × serum creatinine (mg/dL) | |
| Females: 0.85 × above value | |
| Creatinine Clearance (mL/min) | Recommended Maintenance Schedule | |||
|---|---|---|---|---|
|
|
||||
| Greater than 60 | 500 mg every 12 hours | 1 g every 12 hours | 2 g every 12 hours | 2 g every 8 hours |
| 30 to 60 | 500 mg every 24 hours | 1 g every 24 hours | 2 g every 24 hours | 2 g every 12 hours |
| 11 to 29 | 500 mg every 24 hours | 500 mg every 24 hours | 1 g every 24 hours | 2 g every 24 hours |
| Less than 11 | 250 mg every 24 hours | 250 mg every 24 hours | 500 mg every 24 hours | 1 g every 24 hours |
| Continuous Ambulatory Peritoneal Dialysis (CAPD) | 500 mg every 48 hours | 1 g every 48 hours | 2 g every 48 hours | 2 g every 48 hours |
| Hemodialysis* | 1 g on day 1, then 500 mg every 24 hours thereafter | 1 g every 24 hours | ||
In patients undergoing Continuous Ambulatory Peritoneal Dialysis (CAPD), cefepime for injection may be administered at the recommended doses at a dosage interval of every 48 hours (see Table 2).
In patients undergoing hemodialysis, approximately 68% of the total amount of cefepime present in the body at the start of dialysis will be removed during a 3-hour dialysis period. The dosage of cefepime for injection for hemodialysis patients is 1 g on Day 1 followed by 500 mg every 24 hours for the treatment of all infections except febrile neutropenia, which is 1 g every 24 hours.
Cefepime for injection should be administered at the same time each day and following the completion of hemodialysis on hemodialysis days (see Table 2).
Pediatric Patients
Data in pediatric patients with impaired renal function are not available; however, since cefepime pharmacokinetics are similar in adults and pediatric patients [see Clinical Pharmacology (12.3)], changes in the dosing regimen proportional to those in adults (see Tables 1 and 2) are recommended for pediatric patients.
2.4 Preparation of Cefepime for Injection for Intravenous Infusion
Vials
- Constitute the 1 gram or 2 grams vial, of cefepime for injection with the one of the following diluents:
- Sterile Water for Injection
- 0.9% Sodium Chloride Injection
- 5% Dextrose Injection
- 0.5% or 1% Lidocaine Hydrochloride Injection
- Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol
- Dilute the reconstituted solution with one of the following compatible infusion solutions prior to intravenous infusion (Refer to Table 3 below for the amount of diluent to be added to each vial and the amount of the reconstituted solution to be withdrawn):
- 0.9% Sodium Chloride Injection
- 5% and 10% Dextrose Injection
- M/6 Sodium Lactate Injection
- 5% Dextrose and 0.9% sodium Chloride Injection
- Lactated Ringers and 5% Dextrose Injection
- Normosol®-R and Normosol®-M in 5% Dextrose Injection
- Parenteral drugs should be inspected visually for particulate matter before administration. If particulate matter is evident in reconstituted fluids, the drug solution should be discarded.
- Administer the resulting intravenous infusion over approximately 30 minutes.
- Intermittent intravenous infusion with a Y-type administration set can be accomplished with compatible solutions. However, during infusion of a solution containing cefepime, it is desirable to discontinue the other solution.
2.5 Preparation of Cefepime for Injection, USP for Intramuscular Administration
Constitute cefepime for injection vials 1 gram and 2 grams with one of the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride, 5% Dextrose Injection, 0.5% or 1% Lidocaine Hydrochloride, or Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol. Refer to Table 3 below for the amount of diluent to be added to each vial and the amount of reconstituted volume to be withdrawn.
Parenteral drugs should be inspected visually for particulate matter before administration. If particulate matter is evident in reconstituted fluids, the drug solution should be discarded.
| Single-Dose Vials for Intravenous (IV)/Intramuscular (IM) Administration | Amount of Diluent to be added (mL) | Approximate Cefepime Concentration (mg/mL) | Amount of Reconstituted Volume to be Withdrawn (mL) |
|---|---|---|---|
| Cefepime vial content | |||
| 1 g (IV) | 10 | 100 | 10.5 |
| 1 g (IM) | 2.4 | 280 | 3.6 |
| 2 g (IV) | 10 | 160 | 12.5 |
2.6 Compatibility and Stability
Intravenous Cefepime for Injection
Intravenous Infusion Compatibility
Cefepime for injection vials are compatible at concentrations between 1 mg per mL and 40 mg per mL with the following intravenous infusion fluids: 0.9% Sodium Chloride Injection, 5% and 10% Dextrose Injection, M/6 Sodium Lactate Injection, 5% Dextrose and 0.9% Sodium Chloride Injection, Lactated Ringers and 5% Dextrose Injection, Normosol®-R, and Normosol®-M in 5% Dextrose Injection. These solutions may be stored up to 24 hours at controlled room temperature 20°C to 25°C (68°F to 77°F) or 7 days in a refrigerator 2°C to 8°C (36°F to 46°F).
Admixture Compatibility
Cefepime for injection admixture compatibility information is summarized in Table 4.
| Stability Time for | ||||
|---|---|---|---|---|
| Cefepime for injection Concentration | Admixture and Concentration | Intravenous (IV) Infusion Solutions | RT/L (20° to 25°C) | Refrigeration (2° to 8°C) |
| NS = 0.9% Sodium Chloride Injection. D5W = 5% Dextrose Injection. na = not applicable. RT/L = Ambient room temperature and light. |
||||
| 40 mg/mL | Amikacin 6 mg/mL | NS or D5W | 24 hours | 7 days |
| 40 mg/mL | Ampicillin 1 mg/mL | D5W | 8 hours | 8 hours |
| 40 mg/mL | Ampicillin 10 mg/mL | D5W | 2 hours | 8 hours |
| 40 mg/mL | Ampicillin 1 mg/mL | NS | 24 hours | 48 hours |
| 40 mg/mL | Ampicillin 10 mg/mL | NS | 8 hours | 48 hours |
| 4 mg/mL | Ampicillin 40 mg/mL | NS | 8 hours | 8 hours |
| 4 to 40 mg/mL | Clindamycin Phosphate 0.25 to 6 mg/mL | NS or D5W | 24 hours | 7 days |
| 4 mg/mL | Heparin 10 to 50 units/mL | NS or D5W | 24 hours | 7 days |
| 4 mg/mL | Potassium Chloride 10 to 40 mEq/L | NS or D5W | 24 hours | 7 days |
| 4 mg/mL | Theophylline 0.8 mg/mL | D5W | 24 hours | 7 days |
| 1 to 4 mg/mL | na | Aminosyn™ II 4.25% with electrolytes and calcium | 8 hours | 3 days |
| 0.125 to 0.25 mg/mL | na | Inpersol™ with 4.25% dextrose | 24 hours | 7 days |
Cefepime for Injection Admixture Incompatibility
Do not add solutions of cefepime for injection, to solutions of ampicillin at a concentration greater than 40 mg per mL, or to metronidazole, vancomycin, gentamicin, tobramycin, netilmicin sulfate, or aminophylline because of potential interaction. However, if concurrent therapy with cefepime for injection is indicated, each of these antibiotics can be administered separately.
Intramuscular Cefepime for Injection
Cefepime for injection constituted as directed is stable for 24 hours at controlled room temperature 20°C to 25°C (68°F to 77°F) or for 7 days in a refrigerator 2°C to 8°C (36°F to 46°F) with the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride Injection, 5% Dextrose Injection, Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol, or 0.5% or 1% Lidocaine Hydrochloride.
3 DOSAGE FORMS AND STRENGTHS
Cefepime for Injection, USP is a sterile white to pale yellow powder of cefepime in single-dose vials and it is available in the following strengths:
- 1 gram per vial
- 2 grams per vial
4 CONTRAINDICATIONS
Cefepime for injection is contraindicated in patients who have shown immediate hypersensitivity reactions to cefepime or the cephalosporin class of antibiotics, penicillins or other beta-lactam antibiotics.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Before therapy with cefepime for injection is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefepime, cephalosporins, penicillins, or other beta-lactams. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibacterial drugs has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to cefepime for injection occurs, discontinue the drug and institute appropriate supportive measures.
5.2 Neurotoxicity
Serious adverse reactions have been reported including life-threatening or fatal occurrences of the following: encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), aphasia, myoclonus, seizures, and nonconvulsive status epilepticus [see Adverse Reactions (6.2)]. Most cases occurred in patients with renal impairment who did not receive appropriate dosage adjustment. However, some cases of neurotoxicity occurred in patients receiving a dosage adjustment appropriate for their degree of renal impairment. In the majority of cases, symptoms of neurotoxicity were reversible and resolved after discontinuation of cefepime and/or after hemodialysis. If neurotoxicity associated with cefepime therapy occurs, discontinue cefepime and institute appropriate supportive measures.
5.3 Clostridioides difficile Associated Diarrhea
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefepime for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy.
CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Development of Drug-Resistant Bacteria
Prescribing cefepime for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
As with other antimicrobials, prolonged use of cefepime for injection may result in overgrowth of nonsusceptible microorganisms. Repeated evaluation of the patient's condition is essential. Should superinfection occur during therapy, appropriate measures should be taken.
5.5 Drug/Laboratory Test Interactions
Urinary Glucose
The administration of cefepime may result in a false-positive reaction for glucose in the urine when using some methods (e.g. Clinitest™ tablets) [see Drug Interactions (7.1)].
Coombs' Tests
Positive direct Coombs' tests have been reported during treatment with cefepime for injection. In patients who develop hemolytic anemia, discontinue the drug and institute appropriate therapy. Positive Coombs' test may be observed in newborns whose mothers have received cephalosporin antibiotics before parturition.
Prothrombin Time
Many cephalosporins, including cefepime, have been associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy. Prothrombin time should be monitored in patients at risk, and exogenous vitamin K administered as indicated.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in the Warnings and Precautions section and below:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Neurotoxicity [see Warnings and Precautions (5.2)]
- Clostridioides difficile Associated Diarrhea [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials using multiple doses of cefepime, 4137 patients were treated with the recommended dosages of cefepime (500 mg to 2 g intravenous every 12 hours). There were no deaths or permanent disabilities thought related to drug toxicity. Sixty-four (1.5%) patients discontinued medication due to adverse reactions. Thirty-three (51%) of these 64 patients who discontinued therapy did so because of rash. The percentage of cefepime-treated patients who discontinued study drug because of drug-related adverse reactions was similar at daily doses of 500 mg, 1 g, and 2 g every 12 hours (0.8%, 1.1%, and 2%, respectively). However, the incidence of discontinuation due to rash increased with the higher recommended doses.
The following adverse reactions (Table 5) were identified in clinical trials conducted in North America (n=3125 cefepime-treated patients).
| Incidence equal to or greater than 1% | Local adverse reactions (3%), including phlebitis (1.3%), pain and/or inflammation (0.6%)*; rash (1.1%) |
| Incidence less than 1% but greater than 0.1% | Colitis (including pseudomembranous colitis), diarrhea, erythema, fever, headache, nausea, oral moniliasis, pruritus, urticaria, vaginitis, vomiting, anemia |
At the higher dose of 2 g every 8 hours, the incidence of adverse reactions was higher among the 795 patients who received this dose of cefepime. They consisted of rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%), and headache (1%).
The following (Table 6) adverse laboratory changes, with cefepime, were seen during clinical trials conducted in North America.
|
|
|
| Incidence equal to or greater than 1% | Positive Coombs' test (without hemolysis) (16.2%); decreased phosphorus (2.8%); increased Alanine Transaminase (ALT) (2.8%), Aspartate Transaminase (AST) (2.4%), eosinophils (1.7%); abnormal PTT (1.6%), Prothrombin Time (PT) (1.4%) |
| Incidence less than 1% but greater than 0.1% | Increased alkaline phosphatase, Blood Urea Nitrogen (BUN), calcium, creatinine, phosphorus, potassium, total bilirubin; decreased calcium*, hematocrit, neutrophils, platelets, White Blood Cells (WBC) |
A similar safety profile was seen in clinical trials of pediatric patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of cefepime for injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In addition to the adverse reactions reported during the North American clinical trials with cefepime, the following adverse reactions have been reported during worldwide postmarketing experience. Encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), aphasia, myoclonus, seizures, and nonconvulsive status epilepticus have been reported [see Warnings and Precautions (5.2)].
Anaphylaxis including anaphylactic shock, transient leukopenia, neutropenia, agranulocytosis and thrombocytopenia, have been reported.
6.3 Cephalosporin-Class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with cefepime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibacterial drugs:
Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal dysfunction, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, hepatic dysfunction including cholestasis, and pancytopenia.
7 DRUG INTERACTIONS
7.1 Drug/Laboratory Test Interactions
The administration of cefepime may result in a false-positive reaction for glucose in the urine with certain methods. It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no cases of cefepime for injection exposure during pregnancy reported from postmarketing experience or from clinical trials. Available data from published observational studies and case reports over several decades with cephalosporin use in pregnant women have not established drug-associated risks of major birth defects, miscarriage or adverse maternal or fetal outcomes (see Data).
Cefepime was not associated with adverse developmental outcomes in rats, mice, or rabbits when administered parenterally during organogenesis. The doses used in these studies were 1.6 (rats), approximately equal to (mice), and 0.3 times (rabbits) the recommended maximum human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from case-control studies and case reports over several decades have not identified an association with cephalosporin use during pregnancy and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Available studies have methodologic limitations, including small sample size, retrospective data collection, and inconsistent comparator groups.
Animal Data
Cefepime was not embryocidal and did not cause fetal malformations when administered parenterally during the period of organogenesis to rats at doses up to 1000 mg/kg/day, to mice at doses up to 1200 mg/kg/day, or to rabbits at doses up to 100 mg/kg/day. These doses are 1.6 times (rats), approximately equal to (mice), and 0.3 times (rabbits) the maximum recommended clinical dose based on body surface area.
8.2 Lactation
Risk Summary
Cefepime is present in human breast milk at low concentrations (approximately 0.5 mcg/mL) following a single intravenous dose of 1000 mg. A nursing infant consuming approximately 1000 mL of human milk per day would receive approximately 0.5 mg of cefepime per day (see Data). There is no information regarding the effects of cefepime on the breastfed infant or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for cefepime for injection and any potential adverse effects on the breastfed child from cefepime for injection or from the underlying maternal condition.
Data
A pharmacokinetic study was conducted in 9 healthy lactating women to evaluate the concentrations of cefepime in plasma and breast milk following a single intravenous dose of 1000 mg. The mean breast milk concentrations of cefepime during the first 8 hours post-dose were approximately 0.5 mcg/mL and then declined and became undetectable between 12- and 24-hours post-dose. The mean cumulative breast milk excretion of cefepime over 24 hours was 0.01% of the administered dose. The pharmacokinetics of cefepime are similar between lactating and non-lactating women.
8.4 Pediatric Use
The safety and effectiveness of cefepime in the treatment of uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, pneumonia, and as empiric therapy for febrile neutropenic patients have been established in the age groups 2 months up to 16 years. Use of cefepime for injection in these age groups is supported by evidence from adequate and well-controlled studies of cefepime in adults with additional pharmacokinetic and safety data from pediatric trials [see Clinical Pharmacology (12.3)].
Safety and effectiveness in pediatric patients below the age of 2 months have not been established. There are insufficient clinical data to support the use of cefepime for injection in pediatric patients for the treatment of serious infections in the pediatric population where the suspected or proven pathogen is H. influenzae type b. In those patients in whom meningeal seeding from a distant infection site or in whom meningitis is suspected or documented, an alternate agent with demonstrated clinical efficacy in this setting should be used.
8.5 Geriatric Use
Of the more than 6400 adults treated with cefepime for injection in clinical studies, 35% were 65 years or older while 16% were 75 years or older. When geriatric patients received the usual recommended adult dose, clinical efficacy and safety were comparable to clinical efficacy and safety in non-geriatric adult patients.
Serious adverse events have occurred in geriatric patients with renal insufficiency given unadjusted doses of cefepime, including life-threatening or fatal occurrences of the following: encephalopathy, myoclonus, and seizures [see Warnings and Precautions (5.2), Adverse Reactions (6.2)].
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored [see Clinical Pharmacology (12.3), Warnings and Precautions (5.2), Dosage and Administration (2.3)].
8.6 Renal Impairment
Adjust the dose of cefepime for injection in patients with creatinine clearance less than or equal to 60 mL/min to compensate for the slower rate of renal elimination [see Dosage Adjustment in Patients with Renal Impairment (2.3)].
10 OVERDOSAGE
Patients who receive an overdose should be carefully observed and given supportive treatment. In the presence of renal insufficiency, hemodialysis, not peritoneal dialysis, is recommended to aid in the removal of cefepime from the body. Symptoms of overdose include encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, neuromuscular excitability and nonconvulsive status epilepticus [see Warnings and Precautions (5.2), Adverse Reactions (6.2), Dosage and Administration (2.3)].
11 DESCRIPTION
Cefepime for Injection, USP is a semi-synthetic, cephalosporin antibacterial for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-en-3-yl]methyl]-1-methylpyrrolidinum chloride,72-(Z)-(O-methyloxime), monohydrochloride, monohydrate, which corresponds to the following structural formula:
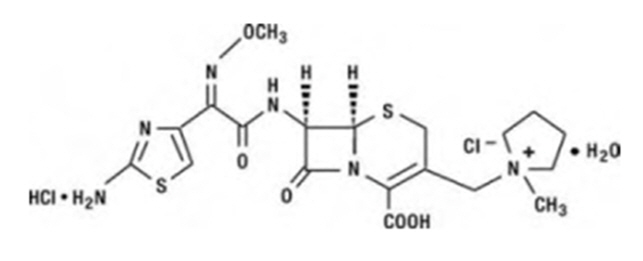
Cefepime hydrochloride is a white to pale yellow powder. Cefepime hydrochloride contains the equivalent of not less than 825 mcg and not more than 911 mcg of cefepime (C19H24N6O5S2) per mg, calculated on an anhydrous basis. It is highly soluble in water.
Cefepime for Injection, USP is supplied for intramuscular or intravenous administration in strengths equivalent to 1 gram and 2 grams of cefepime. Cefepime for Injection, USP is a sterile, dry mixture of cefepime hydrochloride and L-arginine. The L-arginine, at an approximate concentration of 707 mg/g of cefepime, is added to control the pH of the constituted solution at 4 to 6. Freshly constituted solutions of Cefepime for Injection, USP will range in color from pale yellow to amber.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Similar to other beta-lactam antimicrobial agents, the time that the unbound plasma concentration of cefepime exceeds the MIC of the infecting organism has been shown to best correlate with efficacy in animal models of infection. However, the pharmacokinetic/pharmacodynamics relationship for cefepime has not been evaluated in patients.
12.3 Pharmacokinetics
Pharmacokinetic parameters for cefepime in healthy adult male volunteers (n=9) following single 30-minute infusions (IV) of cefepime 500 mg, 1 g, and 2 g are summarized in Table 7. Elimination of cefepime is principally via renal excretion with an average (±SD) half-life of 2 (±0.3) hours and total body clearance of 120 (±8) mL/min in healthy volunteers. Cefepime pharmacokinetics are linear over the range 250 mg to 2 g. There is no evidence of accumulation in healthy adult male volunteers (n=7) receiving clinically relevant doses for a period of 9 days.
| Cefepime for Injection | |||
|---|---|---|---|
| Parameter | 500 mg IV | 1 g IV | 2 g IV |
| Cmax, mcg/mL | 39.1 (3.5) | 81.7 (5.1) | 163.9 (25.3) |
| AUC, h∙mcg/mL | 70.8 (6.7) | 148.5 (15.1) | 284.8 (30.6) |
| Number of subjects (male) | 9 | 9 | 9 |
Pharmacokinetic parameters for cefepime following a single intramuscular injection are summarized in Table 8. The pharmacokinetics of cefepime are linear over the range of 500 mg to 2 g intramuscularly and do not vary with respect to treatment duration.
| Cefepime for Injection | |||
|---|---|---|---|
| Parameter | 500 mg IM | 1 g IM | 2 g IM |
| Cmax, mcg/mL | 13.9 (3.4) | 29.6 (4.4) | 57.5 (9.5) |
| Tmax, h | 1.4 (0.9) | 1.6 (0.4) | 1.5 (0.4) |
| AUC, h∙mcg/mL | 60 (8) | 137 (11) | 262 (23) |
| Number of subjects (male) | 6 | 6 | 12 |
Distribution
The average steady-state volume of distribution of cefepime is 18 (±2) L. The serum protein binding of cefepime is approximately 20% and is independent of its concentration in serum.
Concentrations of cefepime achieved in specific tissues and body fluids are listed in Table 9.
| Tissue or Fluid | Dose/Route | # of Patients | Mean Time of Sample Post-Dose (h) | Mean Concentration |
|---|---|---|---|---|
| Blister Fluid | 2 g IV | 6 | 1.5 | 81.4 mcg/mL |
| Bronchial Mucosa | 2 g IV | 20 | 4.8 | 24.1 mcg/g |
| Sputum | 2 g IV | 5 | 4 | 7.4 mcg/mL |
| Urine | 500 mg IV | 8 | 0 to 4 | 292 mcg/mL |
| 1 g IV | 12 | 0 to 4 | 926 mcg/mL | |
| 2 g IV | 12 | 0 to 4 | 3120 mcg/mL | |
| Bile | 2 g IV | 26 | 9.4 | 17.8 mcg/mL |
| Peritoneal Fluid | 2 g IV | 19 | 4.4 | 18.3 mcg/mL |
| Appendix | 2 g IV | 31 | 5.7 | 5.2 mcg/g |
| Gallbladder | 2 g IV | 38 | 8.9 | 11.9 mcg/g |
| Prostate | 2 g IV | 5 | 1 | 31.5 mcg/g |
Data suggest that cefepime does cross the inflamed blood-brain barrier. The clinical relevance of these data is uncertain at this time.
Metabolism and Excretion
Cefepime is metabolized to N-methylpyrrolidine (NMP) which is rapidly converted to the N-oxide (NMP-N-oxide). Urinary recovery of unchanged cefepime accounts for approximately 85% of the administered dose. Less than 1% of the administered dose is recovered from urine as NMP, 6.8% as NMP-N-oxide, and 2.5% as an epimer of cefepime. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment [see Dosage and Administration (2.3)].
Specific Populations
Patients with Renal impairment
Cefepime pharmacokinetics have been investigated in patients with various degrees of renal impairment (n=30). The average half-life in patients requiring hemodialysis was 13.5 (±2.7) hours and in patients requiring continuous peritoneal dialysis was 19 (±2) hours. Cefepime total body clearance decreased proportionally with creatinine clearance in patients with abnormal renal function, which serves as the basis for dosage adjustment recommendations in this group of patients [see Dosage and Administration (2.3)].
Patients with Hepatic impairment
The pharmacokinetics of cefepime were unaltered in patients with hepatic impairment who received a single 1 g dose (n=11).
Geriatric patients
Cefepime pharmacokinetics have been investigated in elderly (65 years of age and older) men (n=12) and women (n=12) whose mean (SD) creatinine clearance was 74 (±15) mL/min. There appeared to be a decrease in cefepime total body clearance as a function of creatinine clearance. Therefore, dosage administration of cefepime in the elderly should be adjusted as appropriate if the patient's creatinine clearance is 60 mL/min or less [see Dosage and Administration (2.3)].
Pediatric patients
Cefepime pharmacokinetics have been evaluated in pediatric patients from 2 months to 11 years of age following single and multiple doses on every 8 hours (n=29) and every 12 hours (n=13) schedules. Following a single intravenous dose, total body clearance and the steady-state volume of distribution averaged 3.3 (±1) mL/min/kg and 0.3 (±0.1) L/kg, respectively. The urinary recovery of unchanged cefepime was 60.4 (±30.4)% of the administered dose, and the average renal clearance was 2 (±1.1) mL/min/kg. There were no significant effects of age or gender (25 male vs. 17 female) on total body clearance or volume of distribution, corrected for body weight. No accumulation was seen when cefepime was given at 50 mg per kg every 12 hours (n=13), while Cmax, AUC, and t½ were increased about 15% at steady state after 50 mg per kg every 8 hours. The exposure to cefepime following a 50 mg per kg intravenous dose in a pediatric patient is comparable to that in an adult treated with a 2 g intravenous dose. The absolute bioavailability of cefepime after an intramuscular dose of 50 mg per kg was 82.3 (±15)% in eight patients.
12.4 Microbiology
Mechanism of Action
Cefepime is a bactericidal drug that acts by inhibition of bacterial cell wall synthesis. Cefepime has a broad spectrum of in vitro activity that encompasses a wide range of Gram-positive and Gram-negative bacteria. Within bacterial cells, the molecular targets of cefepime are the penicillin binding proteins (PBP).
Antimicrobial Activity
Cefepime has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections as described in the Indications and Usage section (1).
Gram-negative Bacteria
Enterobacter spp.
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Pseudomonas aeruginosa
Gram-positive Bacteria
Staphylococcus aureus (methicillin-susceptible isolates only)
Streptococcus pneumoniae
Streptococcus pyogenes
Viridans group streptococci
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefepime against isolates of similar genus or organism group. However, the efficacy of cefepime in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-positive Bacteria
Staphylococcus epidermidis (methicillin-susceptible isolates only)
Staphylococcus saprophyticus
Streptococcus agalactiae
NOTE: Most isolates of enterococci, e.g., Enterococcus faecalis, and methicillin-resistant staphylococci are resistant to cefepime.
Gram-negative Bacteria
Acinetobacter calcoaceticus subsp. lwoffii
Citrobacter diversus
Citrobacter freundii
Enterobacter agglomerans
Haemophilus influenzae
Hafnia alvei
Klebsiella oxytoca
Moraxella catarrhalis
Morganella morganii
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Serratia marcescens
NOTE: Cefepime is inactive against many isolates of Stenotrophomonas maltophilia.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal carcinogenicity studies have been conducted with cefepime. In chromosomal aberration studies, cefepime was positive for clastogenicity in primary human lymphocytes, but negative in Chinese hamster ovary cells. In other in vitro assays (bacterial and mammalian cell mutation, DNA repair in primary rat hepatocytes, and sister chromatid exchange in human lymphocytes), cefepime was negative for genotoxic effects. Moreover, in vivo assessments of cefepime in mice (2 chromosomal aberration and 2 micronucleus studies) were negative for clastogenicity. No untoward effects on fertility were observed in rats when cefepime was administered subcutaneously at doses up to 1000 mg/kg/day (1.6 times the recommended maximum human dose calculated on a body surface area basis).
14 CLINICAL STUDIES
14.1 Febrile Neutropenic Patients
The safety and efficacy of empiric cefepime monotherapy of febrile neutropenic patients have been assessed in two multicenter, randomized trials comparing cefepime monotherapy (at a dose of 2 g intravenously every 8 hours) to ceftazidime monotherapy (at a dose of 2 g intravenously every 8 hours). These studies comprised 317 evaluable patients. Table 10 describes the characteristics of the evaluable patient population.
| Cefepime | Ceftazidime | |
|---|---|---|
| Total | 164 | 153 |
| ANC = absolute neutrophil count; SBP = systolic blood pressure | ||
| Median age (yr) | 56 (range, 18 to 82) | 55 (range, 16 to 84) |
| Male | 86 (52%) | 85 (56%) |
| Female | 78 (48%) | 68 (44%) |
| Leukemia | 65 (40%) | 52 (34%) |
| Other hematologic malignancies | 43 (26%) | 36 (24%) |
| Solid tumor | 54 (33%) | 56 (37%) |
| Median ANC nadir (cells/microliter) | 20 (range, 0 to 500) | 20 (range, 0 to 500) |
| Median duration of neutropenia (days) | 6 (range, 0 to 39) | 6 (range, 0 to 32) |
| Indwelling venous catheter | 97 (59%) | 86 (56%) |
| Prophylactic antibacterial drugs | 62 (38%) | 64 (42%) |
| Bone marrow graft | 9 (5%) | 7 (5%) |
| SBP less than 90 mm Hg at entry | 7 (4%) | 2 (1%) |
Table 11 describes the clinical response rates observed. For all outcome measures, cefepime was therapeutically equivalent to ceftazidime.
| % Response | ||
|---|---|---|
| Cefepime | Ceftazidime | |
| Outcome Measures | (n=164) | (n=153) |
| Primary episode resolved with no treatment modification, no new febrile episodes or infection, and oral antibiotics allowed for completion of treatment | 51 | 55 |
| Primary episode resolved with no treatment modification, no new febrile episodes or infection and no post-treatment oral antibiotics | 34 | 39 |
| Survival, any treatment modification allowed | 93 | 97 |
| Primary episode resolved with no treatment modification and oral antibiotics allowed for completion of treatment | 62 | 67 |
| Primary episode resolved with no treatment modification and no post-treatment oral antibacterial drugs | 46 | 51 |
Insufficient data exist to support the efficacy of cefepime monotherapy in patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia). No data are available in patients with septic shock.
14.2 Complicated Intra-abdominal Infections
Patients hospitalized with complicated intra-abdominal infections participated in a randomized, double-blind, multicenter trial comparing the combination of cefepime (2 g every 12 hours) plus intravenous metronidazole (500 mg every 6 hours) versus imipenem/cilastatin (500 mg every 6 hours) for a maximum duration of 14 days of therapy. The study was designed to demonstrate equivalence of the two therapies. The primary analyses were conducted on the population consisting of those with a surgically confirmed complicated infection, at least one pathogen isolated pretreatment, at least 5 days of treatment, and a 4 to 6 week follow-up assessment for cured patients. Subjects in the imipenem/cilastatin arm had higher APACHE II scores at baseline. The treatment groups were otherwise generally comparable with regard to their pretreatment characteristics. The overall clinical cure rate among the primary analysis patients was 81% (51 cured/63 evaluable patients) in the cefepime plus metronidazole group and 66% (62/94) in the imipenem/cilastatin group. The observed differences in efficacy may have been due to a greater proportion of patients with high APACHE II scores in the imipenem/cilastatin group.
15 REFERENCES
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31-41.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Cefepime for Injection, USP is supplied as follows:
| NDC | Cefepime for Injection, USP | Package Factor |
|---|---|---|
|
|
||
| 70594-089-02 | 1 gram* Single-Dose Vial | 10 vials per carton |
| 70594-090-02 | 2 gram* Single-Dose Vial | 10 vials per carton |
Cefepime for Injection, USP in the dry state, is a white to pale yellow powder. Constituted solutions of Cefepime for Injection, USP can range in color from pale yellow to amber.
Storage and Handling
Cefepime for Injection, USP in the dry state should be stored at 20° to 25°C (68° to 77°F); excursion permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
Protect from light. Retain in carton until time of use.
Discard unused portion.
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex.
17 PATIENT COUNSELING INFORMATION
- Counsel patients that antibacterial drugs including cefepime for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefepime for injection is prescribed to treat a bacterial infection, tell patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefepime for injection or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibacterial drugs, which usually ends when the antibacterial drug is discontinued. Inform patient that they may develop watery and bloody stools (with or without stomach cramps and fever) during treatment and as late as two or more months after having taken the last dose of the antibacterial drug. Inform patients that they should contact their physician as soon as possible if this occurs.
- Advise patients of neurological adverse events that could occur with cefepime for injection use. Instruct patients or their caregivers to inform their healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), aphasia (disturbance of speaking and understanding spoken and written language), myoclonus, seizures and nonconvulsive status epilepticus, for immediate treatment, dosage adjustment, or discontinuation of cefepime for injection.
Brands listed are the trademarks of their respective owners.
Mfd for Xellia Pharmaceuticals USA, LLC
Buffalo Grove, IL 60089
Made in India
February 2022
3CDG03-02
PRINCIPAL DISPLAY PANEL - 1 gram Vial Carton
NDC: 70594-089-02
Rx Only
Cefepime
for Injection, USP
1 gram per vial
Reconstitute before Intramuscular Use
Dilute before Intravenous Infusion
10 Single-Dose Vials
xellia
PHARMACEUTICALS

PRINCIPAL DISPLAY PANEL - 2 gram Vial Carton
NDC: 70594-090-02
Rx Only
Cefepime
for Injection, USP
2 grams per vial
Dilute before Intravenous Infusion
10 Single-Dose Vials
xellia
PHARMACEUTICALS
| CEFEPIME HYDROCHLORIDE
cefepime hydrochloride injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEFEPIME HYDROCHLORIDE
cefepime hydrochloride injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Xellia Pharmaceuticals USA LLC (116768762) |