LONAZEM- menthol,petrolatum ointment
LONAZEM by
Drug Labeling and Warnings
LONAZEM by is a Otc medication manufactured, distributed, or labeled by PEER PHARM LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
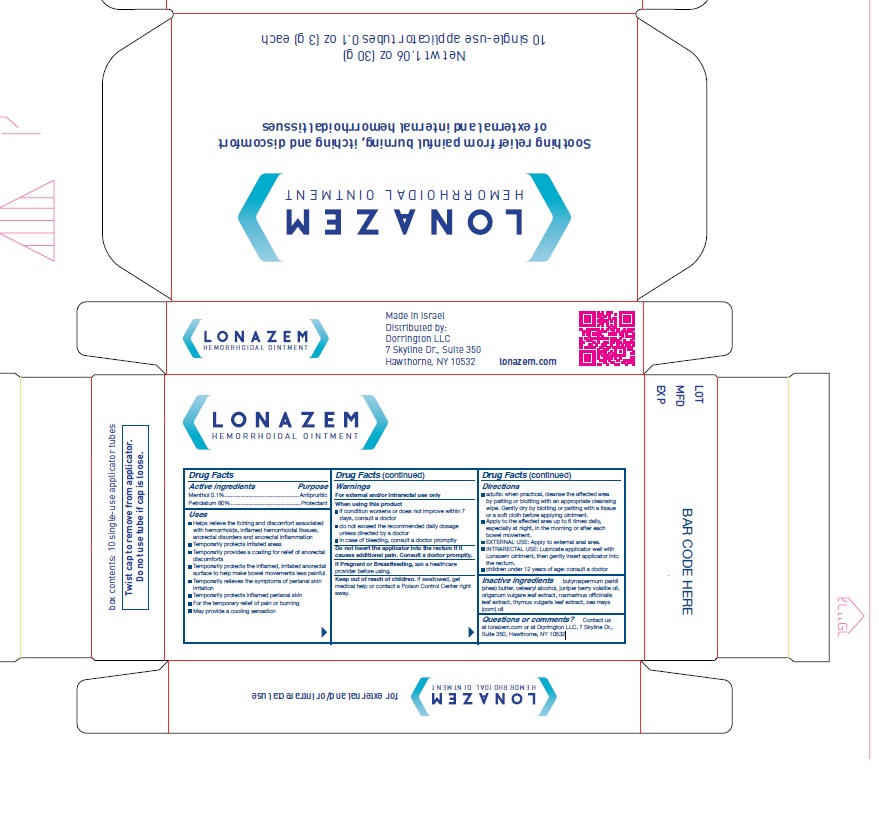
- Active Ingredients
- Purpose
-
Uses
- Helps relieve the itching and discomfort associated with hemorrhoids, inflamed hemorrhoidal tissues, anorectal disorders and anorectal inflammation
- Temporarily protects irritated areas
- Temporarily provides a coating for relief of anorectal discomforts
- Temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
- Temporarily relieves the symptoms of perianal skin irritation
- Temporarily protects inflamed perianal skin
- For the temporary relief of pain or burning
- May provide a cooling sensation
-
Warnings
- For external and/or intrarectal use only
- When using this product
if condition worsens or does not improve within 7 days, consult a doctor
do not exceed the recommended daily dosage unless directed by a doctor
in case of bleeding, consult a doctor promptly- Do not insert the applicator into the rectum if it causes additional pain. Consult a doctor promptly.
If Pregnant or Breastfeeding, ask a healthcare provider before using.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by blotting or patting with a tissue or a soft cloth before applying ointment.
- Apply to the affected area up to 6 times daily, especially at night, in the morning or after ' each bowel movement.
- EXTERNAL USE: Apply to external anal area.
- INTRARECTAL USE: Lubricate applicator well with Lonazem ointment, then gently insert applicator into the rectum.
- Children under 12 years of age: consult a doctor
- Inactive Ingredients
- Questions or comments?
- Product label
-
INGREDIENTS AND APPEARANCE
LONAZEM
menthol,petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69435-1200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 80 g in 100 g Inactive Ingredients Ingredient Name Strength JUNIPER BERRY OIL (UNII: SZH16H44UY) OREGANO (UNII: 0E5AT8T16U) THYMUS VULGARIS LEAF (UNII: GRX3499643) ROSEMARY (UNII: IJ67X351P9) CORN OIL (UNII: 8470G57WFM) SHEA BUTTER (UNII: K49155WL9Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69435-1200-1 10 in 1 CARTON 03/01/2024 1 3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/01/2024 Labeler - PEER PHARM LTD. (514678390) Establishment Name Address ID/FEI Business Operations PEER PHARM LTD. 514678390 manufacture(69435-1200)
Trademark Results [LONAZEM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LONAZEM 86275245 not registered Dead/Abandoned |
PERRIGO PHARMACEUTICALS COMPANY 2014-05-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.