head congestion and mucus by Allwell Health Inc / LNK International, Inc. Allwell Health 44-527C
head congestion and mucus by
Drug Labeling and Warnings
head congestion and mucus by is a Otc medication manufactured, distributed, or labeled by Allwell Health Inc, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
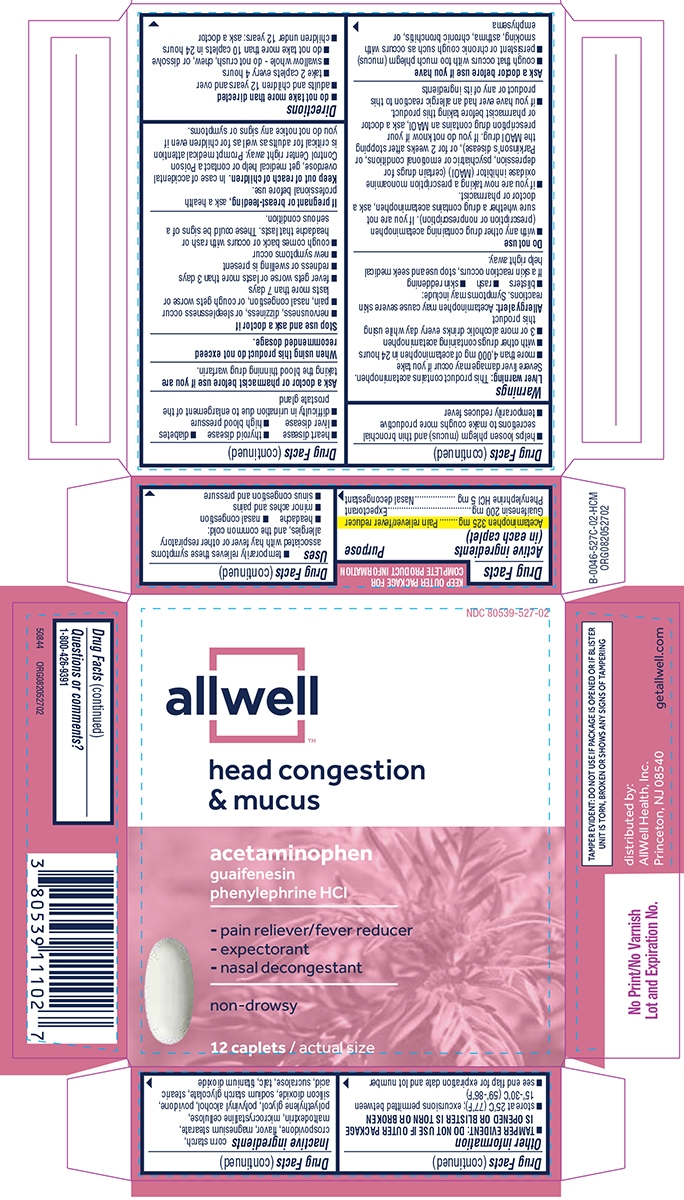
HEAD CONGESTION AND MUCUS- acetaminophen, guaifenesin, phenylephrine hcl tablet, film coated
Allwell Health Inc
----------
Allwell Health 44-527C
Uses
- temporarily relieves these symptoms associated with hay fever or other respiratory allergies, and the common cold:
- headache
- nasal congestion
- minor aches and pains
- sinus congestion and pressure
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- heart disease
- thyroid disease
- diabetes
- liver disease
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Directions
- do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 4 hours
- swallow whole - do not crush, chew, or dissolve
- do not take more than 10 caplets in 24 hours
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, crospovidone, flavor, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, sucralose, talc, titanium dioxide
Principal Display Panel
NDC: 80539-527-02
allwell™
head congestion
& mucus
acetaminophen
guaifenesin
phenylephrine HCl
- pain reliever/fever reducer
- expectorant
- nasal decongestant
non-drowsy
12 caplets / actual size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
distributed by:
AllWell Health, Inc.
Princeton, NJ 08540
getallwell.com
50844 ORG082052702
44-527
| HEAD CONGESTION AND MUCUS
acetaminophen, guaifenesin, phenylephrine hcl tablet, film coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Allwell Health Inc (117644800) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(80539-527) , pack(80539-527) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(80539-527) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(80539-527) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.