JUBLIA- efinaconazole solution
JUBLIA by
Drug Labeling and Warnings
JUBLIA by is a Prescription medication manufactured, distributed, or labeled by Bausch Health US LLC, Kaken Pharmaceutical Co., Ltd., Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JUBLIA safely and effectively. See full prescribing information for JUBLIA.
JUBLIA® (efinaconazole) topical solution, 10%
For topical use
Initial U.S. Approval: 2014INDICATIONS AND USAGE
JUBLIA is an azole antifungal indicated for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. (1)
DOSAGE AND ADMINISTRATION
- Apply JUBLIA to affected toenails once daily for 48 weeks using the integrated flow-through brush applicator. (2)
- When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered. (2)
- For topical use only. (2)
- Not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Solution: 10%. (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
The most common adverse reactions (incidence >1%) were ingrown toenails, application site dermatitis, application site vesicles, and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply JUBLIA to affected toenails once daily for 48 weeks, using the integrated flow-through brush applicator. When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered.
JUBLIA is for topical use only and not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two clinical trials, 1227 subjects were treated with JUBLIA, 1161 for at least 24 weeks and 780 for 48 weeks. Adverse reactions reported within 48 weeks of treatment and in at least 1% of subjects treated with JUBLIA and those reported in subjects treated with the vehicle are presented in Table 1.
Table 1: Adverse Reactions Reported by at Least 1% of Subjects Treated for up to 48 Weeks Adverse Event, n (%)
JUBLIA
N = 1227Vehicle
N = 413Ingrown toenail
28 (2.3%)
3 (0.7%)
Application site dermatitis
27 (2.2%)
1 (0.2%)
Application site vesicles
20 (1.6%)
0 (0.0%)
Application site pain
13 (1.1%)
1 (0.2%)
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies with JUBLIA in pregnant women. JUBLIA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 2, 10 and 50 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-16) to pregnant female rats. In the presence of maternal toxicity, embryofetal toxicity (increased embryofetal deaths, decreased number of live fetuses, and placental effects) was noted at 50 mg/kg/day [559 times the Maximum Recommended Human Dose (MRHD) based on Area Under the Curve (AUC) comparisons]. No embryofetal toxicity was noted at 10 mg/kg/day (112 times the MRHD based on AUC comparisons). No malformations were observed at 50 mg/kg/day (559 times the MRHD based on AUC comparisons).
Subcutaneous doses of 1, 5, and 10 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-19) to pregnant female rabbits. In the presence of maternal toxicity, there was no embryofetal toxicity or malformations at 10 mg/kg/day (154 times the MRHD based on AUC comparisons).
In a pre- and post-natal development study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day efinaconazole were administered from the beginning of organogenesis (gestation day 6) through the end of lactation (lactation day 20). In the presence of maternal toxicity, embryofetal toxicity (increased pre-natal pup mortality, reduced live litter sizes and increased post-natal pup mortality) was noted at 25 mg/kg/day. No embryofetal toxicity was noted at 5 mg/kg/day (17 times the MRHD based on AUC comparisons). No effects on post-natal development were noted at 25 mg/kg/day (89 times the MRHD based on AUC comparisons).
8.3 Nursing Mothers
It is not known whether efinaconazole is excreted in human milk. After repeated subcutaneous administration, efinaconazole was detected in milk of nursing rats. Because many drugs are excreted in human milk, caution should be exercised when JUBLIA is administered to nursing women.
8.4 Pediatric Use
Safety and effectiveness of JUBLIA in pediatric subjects have not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical trials of JUBLIA, 11.3% were 65 and over, while none were 75 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and the younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
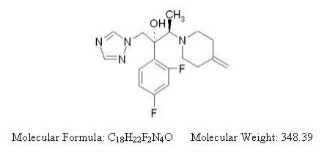
JUBLIA (efinaconazole) topical solution, 10% is a clear colorless to pale yellow solution for topical use. Each gram of JUBLIA contains 100 mg of efinaconazole. Efinaconazole is an azole antifungal with a chemical name of ((2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl) butan-2-ol). The structural formula for efinaconazole is represented below:
JUBLIA contains the following inactive ingredients: alcohol, anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
JUBLIA topical solution is an azole antifungal [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Systemic absorption of efinaconazole in 18 adult subjects with severe onychomycosis was determined after application of JUBLIA once daily for 28 days to patients’ 10 toenails and 0.5 cm adjacent skin. The concentration of efinaconazole in plasma was determined at multiple time points over the course of 24-hour periods on days 1, 14, and 28. Efinaconazole mean ± SD plasma Cmax on Day 28 was 0.67 ± 0.37 ng/mL and the mean ± SD AUC was 12.15 ± 6.91 ng*h/mL. The plasma concentration versus time profile at steady state was generally flat over a 24-hour dosing interval. In a separate study of healthy volunteers, the plasma half-life of efinaconazole following daily applications when applied to all 10 toenails for 7 days was 29.9 hours.
Drug Interactions
JUBLIA is considered a non-inhibitor of the CYP450 enzyme family. In in vitro studies using human liver microsomes, efinaconazole did not inhibit CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2PE1 and CYP3A4 enzyme activities at expected clinical systemic concentrations. In vitro studies in human primary hepatocytes showed that efinaconazole did not induce CYP1A2 or CYP3A4 activities.
12.4 Microbiology
Mechanism of Action
Efinaconazole is an azole antifungal. Efinaconazole inhibits fungal lanosterol 14α-demethylase involved in the biosynthesis of ergosterol, a constituent of fungal cell membranes.
Activity In Vitro and In Vivo
Efinaconazole has been shown to be active against isolates of the following microorganisms, both in vitro and in clinical infections. Efinaconazole exhibits in vitro minimum inhibitory concentrations (MICs) of 0.06 mcg/mL or less against most (≥90%) isolates of the following microorganisms:
Trichophyton rubrum
Trichophyton mentagrophytes
Mechanism of Resistance
Efinaconazole drug resistance development was studied in vitro against T. mentagrophytes, T. rubrum and C. albicans. Serial passage of fungal cultures in the presence of sub-growth inhibitory concentrations of efinaconazole increased the MIC by up to 4-fold. The clinical significance of these in vitro results is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year dermal carcinogenicity study in mice was conducted with daily topical administration of 3%, 10% and 30% efinaconazole solution. Severe irritation was noted at the treatment site in all dose groups, which was attributed to the vehicle and confounded the interpretation of skin effects by efinaconazole. The high dose group was terminated at week 34 due to severe skin reactions. No drug-related neoplasms were noted at doses up to 10% efinaconazole solution (248 times the MRHD based on AUC comparisons).
Efinaconazole revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster lung cell chromosome aberration assay) and one in vivo genotoxicity test (mouse peripheral reticulocyte micronucleus assay).
No effects on fertility were observed in male and female rats that were administered subcutaneous doses up to 25 mg/kg/day efinaconazole (279 times the MRHD based on AUC comparisons) prior to and during early pregnancy. Efinaconazole delayed the estrous cycle in females at 25 mg/kg/day but not at 5 mg/kg/day (56 times MRHD based on AUC comparisons).
-
14 CLINICAL STUDIES
The safety and efficacy of once daily use of JUBLIA for the treatment of onychomycosis of the toenail were assessed in two 52-week prospective, multi-center, randomized, double-blind clinical trials in patients 18 years and older (18 to 70 years of age) with 20% to 50% clinical involvement of the target toenail, without dermatophytomas or lunula (matrix) involvement. The trials compared 48 weeks of treatment with JUBLIA to the vehicle solution. The Complete Cure rate was assessed at Week 52 (4 weeks after completion of therapy). Complete cure was defined as 0% involvement of the target toenail (no clinical evidence of onychomycosis of the target toenail) in addition to Mycologic Cure, defined as both negative fungal culture and negative KOH. Table 2 lists the efficacy results for trials 1 and 2.
- Table 2: Efficacy Endpoints
Trial 1 Trial 2 JUBLIA Vehicle JUBLIA Vehicle N = 656 N = 214 N = 580 N = 201 a Complete cure defined as 0% clinical involvement of the target toenail plus negative KOH and negative culture. b Complete or almost complete cure defined as ≤5% affected target toenail area involved and negative KOH and culture. c Mycologic cure defined as negative KOH and negative culture. Complete Curea
117
17.8%7
3.3%88
15.2%11
5.5%Complete or
Almost Complete Cureb173
26.4%15
7.0%136
23.4%15
7.5%Mycologic Curec
362
55.2%36
16.8%310
53.4%34
16.9% -
16 HOW SUPPLIED/STORAGE AND HANDLING
JUBLIA (efinaconazole) topical solution, 10% is a clear, colorless to pale yellow solution supplied in a white plastic bottle with an integrated flow-through brush applicator as follows:
- 4 mL (NDC: 0187-5400-04)
- 8 mL (NDC: 0187-5400-08)
Storage and Handling Conditions:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
- Solution is flammable; keep away from heat or flame
- Protect from freezing
- Keep out of the reach of children
- Keep bottle tightly closed
- Store in upright position
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- JUBLIA is for external use only and is not for ophthalmic, oral, or intravaginal use. It is for use on toenails and immediately adjacent skin only.
- Apply JUBLIA once daily to clean dry toenails. Wait for at least 10 minutes after showering, bathing, or washing before applying.
- Use JUBLIA only on the affected toenails, as directed by your healthcare provider.
- Inform a healthcare professional if the area of application shows signs of persistent irritation (for example, redness, itching, swelling).
- The impact of nail polish or other cosmetic nail products on the efficacy of JUBLIA has not been evaluated.
- Flammable, avoid use near heat or open flame.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Valeant Pharmaceuticals North America LLCBridgewater, NJ 08807 USA
by:
Valeant Pharmaceuticals International Inc.
Laval, Quebec H7L 4A8, Canada
Or
Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
by:
Kaken Pharmaceutical Co., Ltd.Shizuoka-ken 426-8646, Japan
U.S. Patents 7,214,506; 8,039,494; 8,486,978; and 9,302,009Jublia is a trademark of Valeant Pharmaceuticals
International, Inc. or its affiliates.
© Valeant Pharmaceuticals North America LLC
Valeant Pharmaceuticals International Inc. PI:
Rev. 09/20169462903
Kaken Pharmaceutical Co. Ltd PI:
Rev. 09/20169391906
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
JUBLIA (joo-blee-uh)
(efinaconazole) topical solution, 10%Important information: JUBLIA is for use on toenails and surrounding skin only. Do not use JUBLIA in your mouth, eyes, or vagina.
What is JUBLIA?
JUBLIA is a prescription medicine used to treat fungal infections of the toenails.
It is not known if JUBLIA is safe and effective in children.
What should I tell my healthcare provider before using JUBLIA?
Before you use JUBLIA, tell your healthcare provider about all your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if JUBLIA can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if JUBLIA passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use JUBLIA?
See the “Instructions for Use” at the end of this Patient Information leaflet for detailed information about the right way to use JUBLIA.
- Use JUBLIA exactly as your healthcare provider tells you to use it. Apply JUBLIA to your affected toenails 1 time each day. Wait for at least 10 minutes after showering, bathing, or washing before applying JUBLIA. JUBLIA is used for 48 weeks.
- It is not known if the use of nail polish or other cosmetic nail products (such as gel nails or acrylic nails) will affect how JUBLIA works.
What should I avoid while using JUBLIA?
- JUBLIA is flammable. Avoid heat and flame while applying JUBLIA to your toenail.
What are the possible side effects of JUBLIA?
JUBLIA may cause irritation at the treated site. The most common side effects include: ingrown toenail, redness, itching, swelling, burning or stinging, blisters, and pain. Tell your healthcare provider if you have any side effects that bother you or that do not go away.
These are not all the possible side effects of JUBLIA.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store JUBLIA?
- Store JUBLIA at room temperature, between 68°F to 77°F (20°C to 25°C). Do not freeze JUBLIA.
- Keep the bottle tightly closed and store in an upright position.
- JUBLIA is flammable. Keep away from heat and flame.
Keep JUBLIA and all medicines out of the reach of children.
General information about the safe and effective use of JUBLIA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about JUBLIA that is written for health professionals. Do not use JUBLIA for a condition for which it was not prescribed. Do not give JUBLIA to other people, even if they have the same condition you have. It may harm them.
What are the ingredients in JUBLIA?
Active ingredients: efinaconazole
Inactive ingredients: alcohol, anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water.Manufactured for:
Valeant Pharmaceuticals North America LLCBridgewater, NJ 08807 USA
by:
Valeant Pharmaceuticals International Inc.
Laval, Quebec H7L 4A8, Canada
Or
Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
by:
Kaken Pharmaceutical Co., Ltd.Shizuoka-ken 426-8646, Japan
For more information, call 1-800-321-4576.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Valeant Pharmaceuticals International Inc. Patient Information:
Rev. 09/20169462903
Kaken Pharmaceutical Co. Ltd Patient Information:
Rev. 09/20169391906
Instructions for Use
JUBLIA® (joo-blee-uh)
(efinaconazole)
topical solution, 10%Important information: JUBLIA is for use on toenails and surrounding skin only. Do not use JUBLIA in your mouth, eyes or vagina.
Read this Instructions for Use that comes with JUBLIA before you start using it. Talk to your healthcare provider if you have any questions.
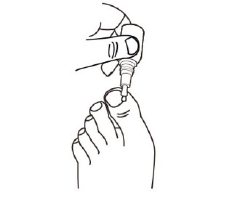
How to apply JUBLIA:
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Manufactured for:
Valeant Pharmaceuticals North America LLC Bridgewater, NJ 08807 USA
Manufactured by: Valeant Pharmaceuticals International, Inc., Laval, Quebec H7L 4A8, Canada
Or
Manufactured for: Valeant Pharmaceuticals North America LLC Bridgewater, NJ 08807 USA
Manufactured by: Kaken Pharmaceutical Co., Ltd., Shizuoka, Japan
Valeant Pharmaceuticals International Inc.
PI and Instructions for Use:
Rev. 09/2016
9462903Kaken Pharmaceutical Co., Ltd.
PI and Instructions for Use:
Rev. 09/2016
9391906 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JUBLIA
efinaconazole solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0187-5400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EFINACONAZOLE (UNII: J82SB7FXWB) (EFINACONAZOLE - UNII:J82SB7FXWB) EFINACONAZOLE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CYCLOMETHICONE (UNII: NMQ347994Z) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-5400-04 1 in 1 CARTON 06/06/2014 1 4 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC: 0187-5400-10 1 in 1 CARTON 06/06/2014 07/24/2019 2 4 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 3 NDC: 0187-5400-08 1 in 1 CARTON 06/06/2014 3 8 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 4 NDC: 0187-5400-02 1 in 1 CARTON 12/17/2015 4 2 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203567 06/06/2014 Labeler - Valeant Pharmaceuticals North America LLC (042230623) Registrant - Valeant Pharmaceuticals North America LLC (042230623) Establishment Name Address ID/FEI Business Operations KAKEN Pharmaceutical Company LTD 706296951 MANUFACTURE(0187-5400) Establishment Name Address ID/FEI Business Operations Valeant Pharmaceuticals International, Inc 245141858 MANUFACTURE(0187-5400) , PACK(0187-5400) Establishment Name Address ID/FEI Business Operations Carton Services, Inc. 928861723 LABEL(0187-5400) , PACK(0187-5400)
Trademark Results [JUBLIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JUBLIA 86414520 4775839 Live/Registered |
BAUSCH HEALTH IRELAND LIMITED 2014-10-03 |
 JUBLIA 85261969 4359772 Live/Registered |
BAUSCH HEALTH IRELAND LIMITED 2011-03-09 |
 JUBLIA 78862951 not registered Dead/Abandoned |
Biovail Laboratories International SRL 2006-04-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.