EVARREST- fibrinogen human and human thrombin patch
EVARREST by
Drug Labeling and Warnings
EVARREST by is a Other medication manufactured, distributed, or labeled by Ethicon, Inc., Omrix Biopharmaceuticals Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EVARREST® safely and effectively. See full prescribing information for EVARREST®.
EVARREST® Fibrin Sealant Patch
Absorbable Patch for Topical Use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
EVARREST® is a fibrin sealant patch indicated for use with manual compression as an adjunct to hemostasis in adult patients undergoing surgery, when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical.
(1)
Limitations for Use:
DOSAGE AND ADMINISTRATION
For topical use only.
- Determine the number of patches to be applied based upon the surface area and anatomic location of the bleeding tissue to be treated. (2)
- Keep the patch dry until use. (2.1)
- Place the powdery (active) side of the patch on the surface of tissue. (2.2)
- Apply immediate manual compression over the entire surface of the patch and maintain contact pressure for 3 minutes to control the bleeding. (2.2)
DOSAGE FORMS AND STRENGTHS
EVARREST® Fibrin Sealant Patch consists of human fibrinogen and human thrombin embedded in a flexible composite patch component. The active side is powdery, and the non-active side has an embossed wave pattern. (3)
Each 2 × 4 inch (5.1 × 10.2 cm) absorbable patch contains
- 55.5 mg per square inch (8.6 mg per square cm) human fibrinogen
- 241.9 Units per square inch (37.5 Units per square cm) human thrombin
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Thrombosis can occur if absorbed systemically. Apply topically to the bleeding site only. (5.1)
- Can cause hypersensitivity reactions including anaphylaxis. (5.2)
- Avoid application to contaminated areas of the body or in the presence of active infection. Infection can occur. (5.3)
- EVARREST® contains oxidized regenerated cellulose which adheres to bleeding surfaces. Inadvertent adhesions can occur. (5.4)
- Avoid use in, around, or in proximity to, foramina in bone or areas of bony confine where swelling may cause compression. (5.5)
- Use the least number of patches required to cover the entire bleeding area. (5.6)
- May carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.7)
ADVERSE REACTIONS
The adverse reactions reported during clinical trials occurred in less than 1% of all cases and included deep venous thrombosis, pulmonary embolism, blood fibrinogen increase, anastomotic hemorrhage, post procedural and intra-abdominal hemorrhage, abdominal distension, anemia, gastrointestinal hemorrhage, thoracic cavity drainage, pleural effusion, abdominal abscess, ascites, localized intra-abdominal fluid collection, cardiac failure, operative hemorrhage, and ischemic bowel. (6)
To report SUSPECTED ADVERSE REACTIONS, contact ETHICON Customer Support Center at 1-877-384-4266 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pediatric: Use in children under the age of one month may be unsafe or ineffective due to small size and limited ability to apply the patch as recommended. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation
2.2 Application
2.3 Retreatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
5.2 Hypersensitivity Reactions
5.3 Infection
5.4 Adhesions
5.5 Compression
5.6 Dislodged Patch Material
5.7 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Safety Data
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and Pharmacology
14 CLINICAL STUDIES
14.1 Abdominal, pelvic, retroperitoneal, and non-cardiac thoracic surgery
14.2 Liver Surgery
14.3 Cardiovascular Surgery
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EVARREST® is a fibrin sealant patch indicated for use with manual compression as an adjunct to hemostasis in adult patients undergoing surgery, when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical.
Limitations for Use:
- Cannot safely or effectively be used in place of sutures or other forms of mechanical ligation in the treatment of major arterial or venous bleeding.
- Not for use in children under one month of age
- Laparoscopic and other minimally invasive surgeries where manual compression would be difficult to achieve.
-
2 DOSAGE AND ADMINISTRATION
For topical use only.
- Determine the number of patches to be applied based upon the surface area and anatomic location of the bleeding to be treated.
- Do not use more than eight 2 × 4 inch (5.1 × 10.2 cm) patches.
- Use in patients who have been previously exposed to EVARREST® has not been studied.
2.1 Preparation
- EVARREST® comes ready to use in sterile packages and must be handled using sterile technique in aseptic conditions. Discard damaged packages as resterilization is not possible.
- To open the product, remove the foil pouch from the carton, carefully peel open the foil pouch, avoiding contact with the inside of the foil or the white sterile tray containing EVARREST.
- Remove the white sterile tray from the pouch and place onto the sterile field.
- Hold the tray securely in the palm of the hand, ensuring that the side with the holes is facing upwards, and use the tabs on the side of the tray to remove the top of the tray with the other hand.
- The lower portion of the tray contains EVARREST with the active side facing downwards. The active side is powdery in appearance. The non-active side has an embossed wave pattern.
- Keep EVARREST dry after opening. The patch can remain in the sterile field to be available for use throughout the procedure. EVARREST does not stick to gloves, forceps, or surgical instruments.
2.2 Application
Apply topically to the bleeding site only.
- Using sterile scissors, carefully cut the patch to the size and shape as necessary to fit and maintain contact with the bleeding area with an overlap of approximately 0.5 to 1 inch (1 to 2 cm). Keep the powdery white-to-yellow color active side of the patch facing down while in the tray.
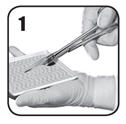
- Remove excess blood or fluid from the site of application to improve visibility. Do not use on non-visualized surfaces. Do not use to treat bleeding from defects in large arteries or veins where the injured vascular wall requires conventional surgical repair for maintenance of vessel patency.
- Apply the active side of the patch to the bleeding area. Allow full contact with the tissue or prosthetic graft. The product is activated upon contact with fluid and then adheres, conforming to tissue with continuous manual compression.

- Apply a sufficient number of patches to adequately cover the entire bleeding area, with an overlap of approximately 0.5 to 1 inch (1 to 2 cm). Use the least number of patches to cover the bleeding area [see Warnings and Precautions (5.6)].
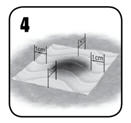
- (a) Hold dry or moist laparotomy pads or surgical gauze over EVARREST® to achieve full contact with the bleeding surface.

(b) To ensure hemostasis, immediately apply continuous manual compression over the entire surface of the patch (including the area of overlap) sufficient to stem all bleeding. Ensure even pressure distribution, full tissue apposition and avoid movement of the patch over the entire bleeding area. Maintain continuous manual compression for 3 minutes.
- Gently remove laparotomy pads or surgical gauze from the application site without disrupting or dislodging EVARREST or the clot. If irrigation is necessary, use care not to dislodge the patch. Inspect the patch to ensure that it is in full contact with the treated area. If placement of the patch is unsatisfactory, remove the patch and use a new patch [see Dosage and Administration (2)]. Removal of the patch may disrupt the clot and result in re-bleeding.
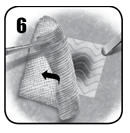
- Discard unused, opened patches at the end of the procedure.
2.3 Retreatment
- Retreatment may be required if there are folds, creases, or crimps in the patch. If not satisfied with the placement of the patch, or if bleeding still occurs during or after the specified duration of compression, remove the used patch and repeat the application procedure above with a new patch.
- If continued bleeding is due to insufficient coverage of the bleeding area, additional patches may be applied. Ensure that the edges overlap (by approximately 0.5 to 1 inch or 1 to 2 cm) with the existing patch.
- If continued bleeding is due to incomplete adherence to the tissue (where bleeding persists from under the dressing), remove the patch and use a new one.
-
3 DOSAGE FORMS AND STRENGTHS
EVARREST® Fibrin Sealant Patch consists of human fibrinogen and human thrombin embedded in a flexible composite patch component. The active side is white-to-yellow in color and powdery in appearance, and the non-active side has an embossed wave pattern.
Each 2 × 4 inch (5.1 × 10.2 cm) absorbable patch of EVARREST contains 55.5 mg per square inch (8.6 mg per square cm) of human fibrinogen and 241.9 Units1 per square inch (37.5 Units per square cm) of human thrombin.
- 1 The Thrombin potency expressed in Units is determined using a clotting assay against an internal reference standard for potency that has been calibrated against the World Health Organization (WHO) Second International Standard for Thrombin, 01/580. Therefore, a Unit used herein is equivalent to an International Unit.
-
4 CONTRAINDICATIONS
Do not use EVARREST® for
- Bleeding from large defects in visible arteries or veins where the injured vascular wall requires conventional surgical repair and maintenance of vessel patency or where there would be persistent exposure of EVARREST to blood flow and/or pressure during absorption of the product.
- Intravascular application. This can result in life-threatening thromboembolic events.
- Individuals known to have anaphylactic or severe systemic reaction to human blood products.
-
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
Thrombosis can occur if EVARREST® is absorbed systemically. Apply EVARREST topically to the bleeding site only.
5.2 Hypersensitivity Reactions
EVARREST® can cause hypersensitivity reactions, including anaphylactic reactions. Symptoms associated with allergic anaphylactic reactions include flush, urticaria, pruritus, nausea, drop in blood pressure, tachycardia or bradycardia, dyspnea, severe hypotension and anaphylactic shock.
5.3 Infection
Avoid application to contaminated or infected areas of the body, or in the presence of active infection. Infection can occur from application to an infected site.
5.4 Adhesions
EVARREST® contains oxidized regenerated cellulose which adheres to bleeding surfaces. Inadvertent adhesions can occur.
5.5 Compression
Avoid using EVARREST® in, around, or in proximity to, foramina in bone or areas of bony confine where swelling may cause nerve or blood vessel compression.
5.6 Dislodged Patch Material
Use the least number of patches possible during surgical procedures because portions of excess patch material can become dislodged and migrate to other areas of the body.
5.7 Transmissible Infectious Agents
Because the biological components of this product are made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk of transmitting viral agents has been minimized by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during the manufacturing process [see Description (11)]. Despite these measures, such products still potentially can transmit disease. There is also the possibility that unknown infectious agents may be present in such products.
Any infection considered by a physician to possibly have been transmitted by this product should be reported by the physician or other healthcare provider to ETHICON Customer Support Center at 1-877-384-4266.
-
6 ADVERSE REACTIONS
All Adverse Reactions reported during the clinical trials occurred at a frequency of less than 1%. Most adverse reactions were reported as single events: intra-abdominal hemorrhage, abdominal distension, anemia, thoracic cavity drainage, pleural effusion, abdominal abscess, ascites, deep vein thrombosis, localized intra-abdominal fluid collection, operative hemorrhage, ischemic bowel and pulmonary embolism except blood fibrinogen increased (3 events, 0.8%) anastomotic hemorrhage (3 events, 0.8%) and post procedural hemorrhage (2 events, 0.5%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates of adverse reactions in clinical trials of another drug and may not reflect the rates observed in clinical practice.
EVARREST® was used to treat soft tissue bleeding during retroperitoneal, intra-abdominal, pelvic, or thoracic surgery, suture hole bleeding during cardiovascular surgery, and parenchymal bleeding during hepatic or renal surgery across all clinical trials involving 381 subjects treated with EVARREST and 272 control subjects. Of the enrolled subjects, 4.7% of EVARREST treated subjects (18 subjects out of 381) and 2.6% of control subjects (7 subjects out of 272) experienced one or more adverse reactions. Table 1 indicates the number of re-bleeding and thromboembolic events reported from each clinical trial.
Table 1. Re-bleeding and Thromboembolic Adverse Reactions Reported in Clinical Trials System Organ Class Preferred term EVARREST All
(n=381)Relationship to Treatment Control
(n=272)Relationship to Treatment Abdominal, pelvic, retroperitoneal, and non-cardiac thoracic surgery Injury, Poisoning and Procedural Complications Operative Hemorrhage 1 (0.3%) Possibly related 2 (0.7%) Related and Possibly related Respiratory, Thoracic and Mediastinal Disorders Pulmonary Embolism 1 (0.3%) Possibly related 0 (0.0%) NA Vascular Disorders Deep Vein Thrombosis 1 (0.3%) Possibly related 0 (0.0%) NA Liver Surgery Gastrointestinal Disorders Intra-Abdominal Hemorrhage 1 (0.3%) Possibly related 0 (0.0%) NA Injury, Poisoning and Procedural Complications Post Procedural Hemorrhage 1 (0.3%) Possibly related 0 (0.0%) NA Renal Surgery Injury, Poisoning and Procedural Complications Post Procedural Hemorrhage 1 (0.3%) Related 0 (0.0%) NA Cardiovascular Surgery Injury, Poisoning and Procedural Complications Anastomotic Hemorrhage 3 (0.8%) 2 × Related; 1 × Possibly related 1 (0.4%) Possibly related Post Procedural Hemorrhage 0 (0.0%) NA 2 (0.7%) Possibly related A prospective, randomized, controlled single-center post-marketing safety study observing the clinical utility of EVARREST against Standard of Care (SoC) in soft tissue bleeding during intra-abdominal, retroperitoneal, pelvic and non-cardiac thoracic surgery was conducted. Standard of Care was manual compression (MC) with or without a topical absorbable hemostat (TAH) or any other adjunctive hemostasis technique that was deemed by the surgeon to be his/her standard of care. The incidence of thromboembolic events, the incidence of post-operative bleeding events specifically related to the target bleeding site and the incidence of increased blood fibrinogen levels were assessed and recorded up to the 30 days (+/- 14 days) follow-up period.
The post-marketing study enrolled 150 subjects and demographics were balanced across the treatment groups. In both groups, no adverse events were assessed as re-bleeding related to target bleeding site; events assessed as thromboembolic events were experienced by 2/75 subjects (2.7%) in the EVARREST group and 7 subjects (9.3%) in the SoC group. One (1/75) adverse reaction of deep vein thrombosis was reported in the EVARREST group. No significant differences were observed in the frequency of adverse reactions between treatment groups.
Immunogenicity was evaluated in soft tissue clinical studies by testing blood samples collected for antibodies to human thrombin and fibrinogen. Three subjects out of 145 (~2%) in the group treated with EVARREST showed an increase in the titer of anti-thrombin antibodies after treatment. Two subjects out of 145 (~1%) in the group treated with EVARREST showed a transient increase in fibrinogen antibody titers, with titer levels back at background levels 8 to 10 weeks after treatment. None of the patients in any treatment group had a significant change in antibody titer to thrombin or fibrinogen. The general antibody level in the treated population was not affected by treatment with EVARREST and resembled the normal levels in healthy subjects.
6.2 Post Marketing Safety Data
No new safety signals were identified from post-marketing experience. There were no reported adverse events that were confirmed to be related to EVARREST. Two cases of post-operative infection were reported when EVARREST was used off-label in a contaminated area. In another two cases, lack of expected efficacy in achieving intraoperative hemostasis was reported in coagulopathic patients: however, because these were spontaneous reports, correct application of the product could not be confirmed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
There are no data with EVARREST® use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with EVARREST. It is not known whether EVARREST can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. EVARREST should be applied to a pregnant woman only if clearly needed.
8.2 Lactation
Risk Summary
There is no information regarding the presence of any component of EVARREST® in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EVARREST and any potential adverse effects on the breastfed infant from EVARREST or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Use of EVARREST® in children under the age of one month may be unsafe or ineffective due to small size and limited ability to apply the patch as recommended. Slow absorption and possibility of adhesions can further complicate use of EVARREST in the neonates.
-
11 DESCRIPTION
EVARREST® Fibrin Sealant Patch is a sterile, bio-absorbable combination product, comprised of two biological components (human plasma-derived fibrinogen and thrombin) embedded in a flexible composite patch component. The active side is white-to-yellow in color and powdery in appearance; the non-active side has an embossed wave pattern.
The patch component of EVARREST consists of an oxidized regenerated cellulose (ORC) layer underlying a layer of polyglactin 910 (PG910) non-woven fibers. The PG910 layer contains the embedded biological components. The patch component provides a large surface area for the biological components and imparts inherent mechanical integrity to the product. The flexibility of EVARREST accommodates the physiological movements of tissues and organs.
Each 2 × 4 inch (5.1 × 10.2 cm) EVARREST patch contains 55.5 mg per square inch (8.6 mg per square cm) of human fibrinogen and 241.9 Units per square inch (37.5 Units per square cm) of human thrombin. Additional inactive ingredients are: arginine hydrochloride, calcium chloride, glycine, human albumin, mannitol, sodium acetate, sodium chloride, and sodium citrate. EVARREST does not contain any preservative.
EVARREST is sterilized by electron-beam irradiation after completion of inner and outer packaging resulting in a sterile product in a sterile inner package.
Viral Clearance
The biological components of EVARREST® (human fibrinogen and human thrombin) are manufactured from pooled human plasma collected in FDA-licensed facilities in the United States. Human plasma is tested by FDA-licensed Nucleic Acid Tests (NAT) for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus-1 (HIV-1). NAT testing for hepatitis A virus (HAV) and parvovirus B19 is also performed. In addition, human plasma is tested for the presence of hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (anti-HCV) and human immunodeficiency viruses types 1 and 2 (anti-HIV1/2).
The manufacturing procedure for human fibrinogen and human thrombin include processing steps designed to reduce the risk of viral transmission. In particular, the virus clearance steps in the manufacture of human fibrinogen include solvent/detergent (S/D) treatment and pasteurization. The virus clearance steps in the manufacture of human thrombin include S/D treatment and nanofiltration.
The virus clearance capacity of these procedures has been validated using viruses with a range of physico-chemical characteristics. These in vitro validation studies were conducted using samples from manufacturing intermediates spiked with virus suspensions of known titers followed by further processing under conditions equivalent to those in the respective manufacturing steps. The results of virus clearance validation studies are summarized in Table 2 and Table 3.
Table 2. Virus Reduction Factors for Human Fibrinogen Reduction Factor (log10) of virus tested * Enveloped Viruses † Non-Enveloped Viruses Manufacturing Step HIV-1 BVDV PRV EMCV HAV CPV - * HIV-1: Human Immunodeficiency Virus Type 1
BVDV: Bovine Viral Diarrhea Virus
PRV: Pseudorabies Virus- † EMCV: Encephalomyocarditis Virus
HAV: Hepatitis A Virus
CPV: Canine ParvovirusSD Treatment > 4.42 > 4.39 > 3.96 Not Tested Not Tested 0.0 Pasteurization > 4.39 > 5.46 6.04 3.69 > 5.78 1.33 Cumulative Virus Reduction Factor > 8.81 > 9.85 > 10.00 3.69 > 5.78 1.33 Table 3. Virus Reduction Factors for Human Thrombin Reduction Factor (log10) of virus tested * Enveloped Viruses † Non-Enveloped Viruses Manufacturing Step HIV-1 SBV BVDV PRV EMCV HAV CPV - * HIV-1: Human Immunodeficiency Virus Type 1
SBV: Sindbis Virus
BVDV: Bovine Viral Diarrhea Virus
PRV: Pseudorabies Virus- † EMCV: Encephalomyocarditis Virus
HAV: Hepatitis A Virus
CPV: Canine ParvovirusSD Treatment > 5.82 > 5.31 > 4.74 > 4.25 Not Tested Not Tested 0.0 Nanofiltration > 4.36 > 5.32 Not Tested > 5.47 6.37 6.95 5.85 Cumulative Virus Reduction Factor > 10.18 > 10.63 > 4.74 > 9.72 6.37 6.95 5.85 - * HIV-1: Human Immunodeficiency Virus Type 1
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of EVARREST® is based on the interaction between the biological components and the physiology of the fibrin clot formation. Upon contact with a bleeding wound surface, the biological components embedded in the patch component are hydrated, and the subsequent fibrinogen-thrombin reaction initiates the last step in the cascade of biochemical reactions - conversion of fibrinogen into fibrin monomers that further polymerize to form the fibrin clot.
Hemostasis is achieved when the formed fibrin clot integrates with the patch component and adheres to the wound surface thus also providing a physical barrier to bleeding.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of EVARREST® or studies to determine the effects of EVARREST on genotoxicity or fertility have not been performed. An assessment of the carcinogenic potential of EVARREST was completed to demonstrate minimal carcinogenic risk from product use.
13.2 Animal Toxicology and Pharmacology
The biological components of EVARREST are degraded by fibrinolysis and phagocytosis, similarly to endogenous fibrin. As resorption progresses, increased fibrinolytic activity is induced by plasmin, and decomposition of fibrin to fibrin degradation products is initiated. In swine and rodent models, topically applied EVARREST® was absorbed at approximately 8 weeks after application with less than 10% of the initial material remaining, which further degraded exponentially over time. In a rodent repeat-dosing model study, EVARREST was subcutaneously applied on Day 0 at twenty times the clinical dose at multiple surgical sites, or on Day 0 and 14 at ten times the clinical dose within multiple surgical sites. There was evidence of resorption at the site or sites of implantation after both single and repeated applications of EVARREST at study termination 90 days later. Repeat use of EVARREST in a rodent model was not associated with any increased local or systemic toxicological effects when used in multiple sites within the same procedure or when used in two procedures conducted 2-weeks apart at separate sites.
-
14 CLINICAL STUDIES
14.1 Abdominal, pelvic, retroperitoneal, and non-cardiac thoracic surgery
A prospective, randomized controlled trial including 141 subjects (median age: 62 years; range 26 to 89 years) was performed to compare the safety and hemostatic effectiveness of EVARREST® with that of oxidized regenerated cellulose (ORC), an absorbable hemostat, when used as an adjunct to control bleeding after primary methods to achieve hemostasis (e.g., suture, cautery, ligature) proved ineffective or impractical during open abdominal, pelvic, retroperitoneal, and non-cardiac thoracic surgery.
A total of 90 subjects were randomized in a 2:1 ratio (60 treated with EVARREST, 30 treated with ORC). An additional 51 subjects were enrolled and treated with EVARREST during a subsequent non-randomized phase. Of the total 141 enrolled subjects, 63.1% were male; 80.1% were White/Caucasian; 18.4% were Black; 0.7% were Asian; and 0.7% were Hispanic/Latino. Median body mass index was 27 kg/m2 (range: 17 to 53 kg/m2). Demographic characteristics were balanced across treatment groups.
EVARREST demonstrated a statistically significant difference compared with ORC, 98.3% versus 53.3% (p < 0.0001) in the proportion of subjects achieving hemostatic success at 4 minutes after identification of the target bleeding site and randomization, with no re-bleeding requiring treatment during a subsequent 6-minute observation period and sustained hemostasis until fascial closure at the end of the surgery (Table 4).
Table 4. Subjects Achieving Hemostasis at 4 Minutes from Identification of the Target Bleeding Site and Randomization, with no Re-bleeding until Fascial Closure at the End of the Surgery EVARREST® ORC p-value Treatment Difference 95% Confidence Interval 59/60 (98.3%) 16/30 (53.3%) <0.0001 45.0% 27.9%, 62.5% Efficacy data for the 51 non-randomized subjects treated with EVARREST were supportive of the above findings, with 50/51 subjects (98.0%) achieving hemostatic success at 4 minutes after randomization. No re-bleeding requiring treatment occurred during a subsequent 6-minute observation period.
14.2 Liver Surgery
Two prospective, randomized controlled trials including a total of 206 subjects were performed to compare the safety and hemostatic effectiveness of EVARREST® with that of Standard of Care (defined as manual compression with or without a topical absorbable hemostat) when used as an adjunct to control bleeding after primary methods to achieve hemostasis (e.g., suture, cautery, ligature) proved ineffective or impractical during liver surgery.
The first study included 104 subjects (median age: 65 years; range 31 to 82 years): 58.7% of the subjects were male; 95.2% were White/Caucasian; 1.9% were Asian; and 1% were Black. Median body mass index was 27kg/m2 (range: 15 to 43 kg/m2). Demographic characteristics were balanced across treatment groups.
A total of 84 subjects were randomized in a 1:1 ratio (40 randomized to EVARREST, 44 to Standard of Care). An additional 20 subjects were enrolled and treated with EVARREST during a non-randomized phase. One subject should have been randomized to EVARREST but was treated with Standard of Care. This subject was analyzed in the EVARREST group for the Intent to Treat Set.
EVARREST demonstrated a statistically significant difference compared with Standard of Care (82.5% versus 29.5%; p < 0.0001) in the proportion of subjects achieving hemostatic success at 4 minutes after identification of the target bleeding site and randomization, with no re-bleeding requiring treatment any time prior to initiation of wound closure (Table 5). The treatment difference between EVARREST and Standard of Care was greater in subjects with bleeding from abnormal hepatic tissue (e.g., cirrhotic or steatotic) than in subjects with normal tissue bleeding (65.2% versus 48.8%, respectively).
Table 5. Subjects Achieving Hemostasis at 4 Minutes from Identification of the Target Bleeding Site and Randomization, with no Re-bleeding until Fascial Closure at the End of the Surgery EVARREST® Standard of Care p-value Treatment Difference 95% Confidence Interval 33/40 (82.5%) 13/44 (29.5%) <0.0001 53.0% 32.9%, 68.5% Efficacy data for the 20 non-randomized subjects treated with EVARREST were supportive of the above findings, with 20/20 subjects (100.0%) achieving hemostatic success at 4 minutes after identification of the target bleeding site and randomization. No re-bleeding requiring treatment occurred any time prior to the initiation of wound closure.
The second study included 102 subjects (median age: 63 years; range 23 to 85 years) randomized in a 1:1 ratio (50 randomized to EVARREST, 52 to Standard of Care). Sixty-one percent of the subjects were male; 85.3% were White/Caucasian; 9.8% were Black; 2.9% were Asian; and 3% were Hispanic. Median body mass index was 27kg/m2 (range: 15 to 43 kg/m2). Demographic characteristics were balanced across the treatment groups.
EVARREST demonstrated a statistically significant difference compared with Standard of Care (96% versus 46.2%; p < 0.0001) in the proportion of subjects achieving hemostatic success at 4 minutes after identification of the target bleeding site and randomization. No re-bleeding requiring treatment occurred at any time prior to initiation of wound closure (Table 6). The treatment difference between EVARREST and Standard of Care was shown to be greater in subjects undergoing non-anatomic resection than in subjects undergoing anatomic resection (58.1% versus 39.5%, respectively). Data from this study further support the efficacy of EVARREST as an adjunct to hemostasis in the control of bleeding in liver surgery.
Table 6. Subjects Achieving Hemostasis at 4 Minutes from Identification of the Target Bleeding Site and Randomization, with no Re-bleeding until Fascial Closure at the End of the Surgery EVARREST® Standard of Care p-value Treatment Difference 95% Confidence Interval 48/50 (96.0%) 24/52 (46.2%) <0.0001 49.8% 34.5%, 63.5% 14.3 Cardiovascular Surgery
Two prospective, randomized controlled trials enrolling a total of 198 subjects were performed to evaluate the safety and efficacy of EVARREST® as an adjunct to control of bleeding during cardiovascular surgery in subjects undergoing major aortic reconstruction using a synthetic graft (with the exception of ePTFE grafts) while on cardiopulmonary bypass and prior to heparin reversal. The target bleeding site was located on the tissue-graft or graft-graft anastomosis.
The first study included 42 subjects randomized 1:1:1 (13 randomized to EVARREST, 18 to TachoSil®, and 11 to Standard of Care). Median age was 56 years in the EVARREST cohort, 60 years in the TachoSil® Fibrin Sealant Patch (TachoSil®) cohort and 61 years in the Standard of Care cohort (range: 28-83 years). Standard of Care was defined as manual compression with or without a topical absorbable hemostat. The number of males exceeded the number of females (69.2% EVARREST, 83.3% TachoSil® and 81.8% Standard of Care) and most were White/Caucasian (69.2% EVARREST, 61.1% TachoSil® and 54.5% Standard of Care). Other demographic characteristics were balanced across treatment cohorts. The majority of subjects had a body mass index (BMI) categorized as "overweight" (BMI: 25 – <30 kg/m2), "obese" (BMI: 30 – <40 kg/m2) or "morbidly obese" (BMI ≥ 40 kg/m2). These categories combined represented 61.6% of EVARREST subjects, 61.2% of TachoSil® subjects and 63.7% of Standard of Care subjects.
Table 7 shows a significant difference in hemostasis between EVARREST and TachoSil® (92.3% versus 33.3%; 2.77; 95% CI 1.53, 5.74) and between EVARREST and Standard of Care (92.3% versus 45.5%; 2.03; 95% CI 1.18, 4.39).
Table 7. Hemostasis at 3 Minutes with No Re-bleeding Until Initiation of Final Chest Wall Closure Treatment Comparison Hemostatic Efficacy Rates (%) Risk Ratio (RR) 95% CI* for RR - * Using Koopman's method
EVARREST® to TachoSil® 92.3% (12/13) 33.3% (6/18) 2.77 (1.53, 5.74) EVARREST® to SoC 92.3% (12/13) 45.5% (5/11) 2.03 (1.18, 4.39) The second study included 156 subjects randomized 1:1 (76 randomized to EVARREST, 80 to TachoSil®). In both treatment cohorts, there were more males than females (75% EVARREST; 75% TachoSil®). Median age was 66 years in the EVARREST cohort and 64 years in the TachoSil® cohort (range: 24-83 years). The majority of subjects were White/Caucasian (82.9% EVARREST; 81. 3% TachoSil®). Median BMI in the EVARREST cohort was 27.1 kg/m2 (range 17.7 – 44.6 kg/m2); 71.0% of subjects were categorized as "overweight" (BMI 25 – <30 kg/m2), "obese" (BMI 30 – <40 kg/m2) or "morbidly obese" (BMI ≥ 40 kg/m2). Median BMI in the TachoSil® cohort was 27.9 kg/m2(range 18.5 – 54.9 kg/m2); 77.6% of subjects were categorized as "overweight", "obese" or "morbidly obese".
Table 8 shows that EVARREST was superior to TachoSil® in the proportion of subjects achieving hemostasis at the target anastomotic bleeding site at 3 minutes after treatment application and with no re-bleeding requiring treatment until chest wall closure (75.0% versus 45.0%; p = 0.0001). Data from this study support the efficacy of EVARREST as an adjunct to hemostasis in the control of bleeding in cardiovascular surgery.
Table 8. Subjects Achieving Hemostasis at 3 Minutes Following Treatment Application, With No Re-Bleeding Up Until Chest Wall Closure EVARREST® TachoSil® p-value Treatment Difference 57/76 (75.0%) 36/80 (45.0%) 0.0001 30.0% -
16 HOW SUPPLIED/STORAGE AND HANDLING
EVARREST® is packaged in a polyester tray and lid assembly within an outer pouch composed of polyester laminated aluminum foil with an inner heat seal coating. The tray and lid assembly maintains product integrity during storage and transport. The aluminum foil pouch serves as a barrier to moisture and microbial contamination to maintain product sterility. Each aluminum foil pouch is contained in a labeled cardboard package.
Each individual package contains two 2 × 4 inch (5.1 × 10.2 cm) EVARREST patches (NDC: 63713-050-25 per patch and NDC: 63713-050-24 per package).
- Use EVARREST before the expiration date indicated on the carton.
- Store unopened packages of EVARREST at 2 to 25°C. EVARREST does not require refrigeration. Do not freeze.
- Do not use if package is opened or damaged.
- Once opened, keep EVARREST dry to avoid activation of the biological components prior to use.
-
17 PATIENT COUNSELING INFORMATION
- Advise patients to consult their physician if they experience chest pain, shortness of breath, difficulty speaking or swallowing, leg tenderness or swelling, or other symptoms of thromboembolism.
- Inform patients that EVARREST® may carry a risk of transmitting infectious agents, e.g., viruses such as hepatitis A and parvovirus B19 and theoretically the CJD agent. Instruct patients to consult their physician if symptoms of B19 virus infection (fever, drowsiness, and chills, followed two weeks later by a rash and joint pain) or hepatitis A (several days to weeks of poor appetite, fatigue and low-grade fever followed by nausea, vomiting and abdominal pain, dark urine, yellowed complexion) appear.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1 Patch Tray Label
NDC: 63713-050-25
FIBRIN SEALANT
PATCH
EVARREST®Contents:
1 Absorbable Fibrin Sealant Patch
Human Fibrinogen: 55.5 mg/in2 (8.6 mg/cm2)
Human Thrombin: 241.9 Units/in2 (37.5 Units/cm2)
Oxidized Regenerated Cellulose, Polyglactin 910Batch No:
Exp Date:
Serial No:FPO
2D
BARCODE
14mm x 14mmManufactured by:
Omrix Biopharmaceuticals Ltd.
14 Einstein Street
Weizmann Science Park
Nes-Ziona, IsraelMade in Israel
Distributed by:
Ethicon, Inc.
Route 22 West, PO Box 151
Somerville, NJ
08876-0151 USAU.S. License No. 1879
Rx only
STERILE R
Art. No.: 59XZ24Z3S2
U.S. Patent 7666803REF EVT5024
2 x 4
inch inch
(5.1cm x 10.2cm)Store at 2°C-25°C (36°F-77°F).
Do not use if package
is opened or damaged.Do not resterilize.
Topical use only
Single use onlyActive side is powdery;
non-active side has an
embossed wave pattern.
-
PRINCIPAL DISPLAY PANEL - 2 Patch Pouch Carton
NDC: 63713-050-24
FIBRIN SEALANT
PATCH
EVARREST®Contents:
2 Absorbable Fibrin Sealant PatchesHuman Fibrinogen: 55.5 mg/in2 (8.6 mg/cm2)
Human Thrombin: 241.9 Units/in2 (37.5 Units/cm2)
Oxidized Regenerated Cellulose, Polyglactin 910
For dosage and inactive ingredients,
see Prescribing InformationNo U.S. standard of potency
Contains no preservativeRx Only
Topical use only
Single use only2 x 4
inch inch
(5.1cm x 10.2cm)2 UNITS
Read the instructions
before use.Store at 2°C-25°C (36°F-77°F).
Do not freeze.Do not use if package is
opened or damaged.
Do not resterilize.Active side is powdery;
non-active side has an
embossed wave pattern.STERILE R
REF EVT5024
-
INGREDIENTS AND APPEARANCE
EVARREST
fibrinogen human and human thrombin patchProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 63713-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 8.6 mg in 1 cm2 Human Thrombin (UNII: 6K15ABL77G) (Human Thrombin - UNII:6K15ABL77G) Human Thrombin 37.5 [iU] in 1 cm2 Inactive Ingredients Ingredient Name Strength Arginine hydrochloride (UNII: F7LTH1E20Y) Calcium chloride (UNII: M4I0D6VV5M) Glycine (UNII: TE7660XO1C) Albumin Human (UNII: ZIF514RVZR) Mannitol (UNII: 3OWL53L36A) Sodium acetate (UNII: 4550K0SC9B) Sodium chloride (UNII: 451W47IQ8X) Sodium citrate, unspecified form (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63713-050-24 2 in 1 CARTON 1 NDC: 63713-050-25 1 in 1 POUCH 1 52 cm2 in 1 TRAY; Type 5: Device Coated or Otherwise Combined with Biologic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125392 03/01/2013 Labeler - Ethicon, Inc. (002144145) Establishment Name Address ID/FEI Business Operations Omrix Biopharmaceuticals Ltd. 514577949 API MANUFACTURE(63713-050) , ANALYSIS(63713-050) Establishment Name Address ID/FEI Business Operations Omrix Biopharmaceuticals Ltd. 649124047 MANUFACTURE(63713-050) , ANALYSIS(63713-050) , PACK(63713-050)
Trademark Results [EVARREST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EVARREST 85758636 4585142 Live/Registered |
JOHNSON & JOHNSON 2012-10-19 |
 EVARREST 77671639 4225953 Live/Registered |
Johnson & Johnson 2009-02-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.