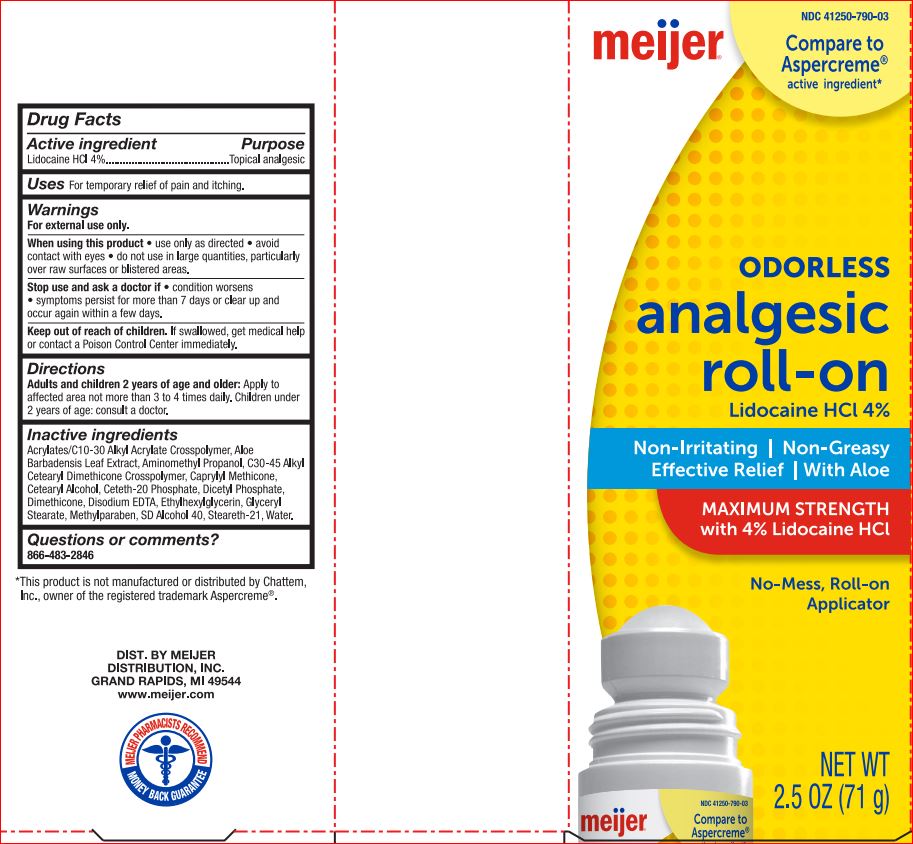
ODORLESS ANALGESIC ROLL-ON MEIJER- lidocaine hcl 4% gel
Odorless Analgesic Roll-on by
Drug Labeling and Warnings
Odorless Analgesic Roll-on by is a Otc medication manufactured, distributed, or labeled by Meijer, Product Quest Mfg. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
When using this product use only as directed avoid contact with eyes do not use in large quantities, particularly over raw surfaces or blistered areas.
Stop use and ask a doctor if condition worsens symptoms persist for more than 7 days or clear up and occur again within a few days - KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis
Leaf Juice, Aminomethyl Propanol, C30-45 Alkyl Cetearyl
Dimethicone Crosspolymer, Caprylyl Methicone, Cetearyl Alcohol,
Ceteth-20 Phosphate, Dicetyl Phosphate, Dimethicone, Disodium
EDTA, Ethylhexylglycerin, Glyceryl Stearate, Methylparaben, SD
Alcohol 40, Steareth-21, Water. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ODORLESS ANALGESIC ROLL-ON MEIJER
lidocaine hcl 4% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41250-790 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 4 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Ceteth-20 Phosphate (UNII: 921FTA1500) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) Dimethicone (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) Ethylhexylglycerin (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Methylparaben (UNII: A2I8C7HI9T) ALCOHOL (UNII: 3K9958V90M) Steareth-21 (UNII: 53J3F32P58) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41250-790-03 71 g in 1 BOTTLE; Type 0: Not a Combination Product 07/11/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/11/2018 Labeler - Meijer (006959555) Registrant - Product Quest Mfg (927768135) Establishment Name Address ID/FEI Business Operations Product Quest Mfg 927768135 manufacture(41250-790) , label(41250-790)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.