GUAIFENESIN AND DEXTROMETHORPHAN ORAL SOLUTION- guaifenesin dextromethorphan hydrobromide oral solution solution
Guaifenesin and Dextromethorphan Oral Solution by
Drug Labeling and Warnings
Guaifenesin and Dextromethorphan Oral Solution by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals, Plastikon Healthcare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Guaifenesin Dextromethorphan hydrobromide 100/10 mg/5 mL Cup
 NCD 0904-6844-70
NCD 0904-6844-70
Guaifenesin and Detromethorphan Oral Solution
100-10 mg/5 mL
Delivers 5 mL
See Insert
For Institutional Use Only
MAJOR® PHARMACEUTICALS
Livonia, MI 48152
Sugar Free - Alcohol Free

Guaifenesin Dextromethorphan hydrobromide 100/10 mg/5 mL cup
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
Age (yr)
Dose (mL)
adults and children 12 years and over
10 mL every 4 hours or 15 mL every 6 hours
Max dose is 60 mL in 24 hr
children 6 years to under 12 years
5 mL every 4 hours
Max does is 30 mL in 24 hr
under 6 years
Ask a doctor
Guaifenesin Dextromethorphan hydrobromide 100/10 mg/5 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Guaifenesin Dextromethorphan hydrobromide 100/10 mg/5 mL
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) or for 2 weeks after stopping the MAOI drug.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
_________________________________________________________________
Ask a doctor or pharmacist before use if you are taking any other drug. ___________________________________________________________________
Stop use and ask a doctor if
- your cough lasts more than 7 days, comes back, or is accompanied by fever, rash or persistent headache.
Guaifenesin Dextromethorphan hydrobromide 100/10 mg/ 5 mL
Inactive ingredients citric acid, glycerin, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol, sucralose, xanthan gum
Guaifenesin Dextromethorphan hydrobromide 100/10 mg/ 5 mL
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold.
-
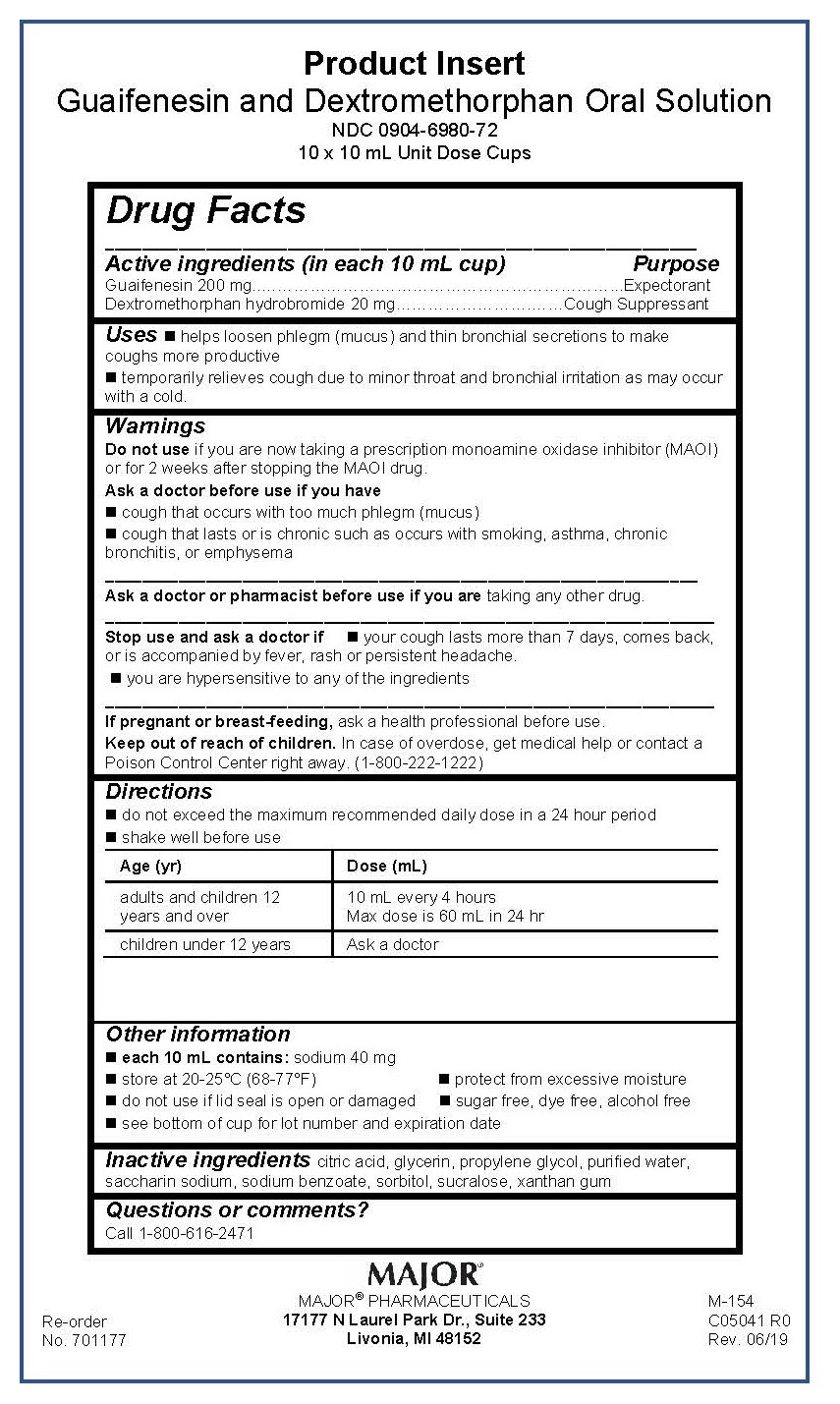
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/10mL

 NCD 0904-6980-72
NCD 0904-6980-72
Guaifenesin and Detromethorphan Oral Solution
200-20 mg/10 mL
Delivers 10 mL
See Insert
For Institutional Use Only
MAJOR® PHARMACEUTICALS
Livonia, MI 48152
Sugar Free - Alcohol Free
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/ 10 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
Age (yr)
Dose (mL)
adults and children 12 years and over
10 mL every 4 hours
Max dose is 60 mL in 24 hr
children under 12 years
Ask a doctor
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/10 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/10 mL
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) or for 2 weeks after stopping the MAOI drug.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
_________________________________________________________________
Ask a doctor or pharmacist before use if you are taking any other drug. ___________________________________________________________________
- Stop use and ask a doctor if
- your cough lasts more than 7 days, comes back, or is accompanied by fever, rash or persistent headache.
- you are hypersensitive to any of the ingredients
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
Guaifenesin Dextromethorpan hydrobromide 200/20 mg/10 mL
Inactive ingredients citric acid, glycerin, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol, sucralose, xanthan gum
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/10 mL
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold.
Guaifenesin Dextromethorphan hydrobromide 200/20 mg/10 mL
Active ingredients (in each 10 mL cup)
Guaifenesin 200 mg
Dextromethorphan hydrobromide 20 mg
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND DEXTROMETHORPHAN ORAL SOLUTION
guaifenesin dextromethorphan hydrobromide oral solution solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6844 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6844-70 10 in 1 CARTON 09/20/2019 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/20/2019 GUAIFENESIN AND DEXTROMETHORPHAN ORAL SOLUTION
guaifenesin dextromethorphan hydrobromide oral solution solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6980 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 10 mL Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6980-72 10 in 1 CARTON 09/20/2019 1 10 in 1 TRAY 1 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/20/2019 Labeler - Major Pharmaceuticals (191427277) Registrant - Plastikon Healthcare, LLC (041717941) Establishment Name Address ID/FEI Business Operations Plastikon Healthcare, LLC 041717941 manufacture(0904-6980, 0904-6844)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.