MANGANESE SULFATE injection, solution
Manganese Sulfate by
Drug Labeling and Warnings
Manganese Sulfate by is a Prescription medication manufactured, distributed, or labeled by American Regent, Inc., Luitpold Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Manganese Sulfate Injection, USP is a sterile, nonpyrogenic solution intended for use as an additive to solutions for Total Parenteral Nutrition (TPN). Each mL contains 0.308 mg of Manganese Sulfate Monohydrate, USP, Water for Injection q.s. pH adjusted with Sulfuric Acid. It delivers elemental manganese 0.1 mg/mL. It is a single dose preservative free vial. Discard any unused portion.
-
CLINICAL PHARMACOLOGY
Manganese is an activator for enzymes such as polysaccharide polymerase, liver arginase, cholinesterase and pyruvate carboxylase. Providing manganese during TPN prevents development of the following deficiency symptoms: nausea and vomiting, weight loss, dermatitis and changes in growth and color of hair.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
Manganese is eliminated via the bile. The possibility of manganese retention should be considered in patients with biliary tract obstruction. Decreasing or omitting manganese supplements entirely may be necessary for such patients. However, ancillary routes of manganese excretion include pancreatic juice, or reabsorption into the lumen of the duodenum, jejunum or ileum.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Manganese Sulfate Injection, USP provides 0.1 mg manganese/mL. For the metabolically stable adult receiving TPN, the suggested additive dosage level for manganese is 0.15 to 0.8 mg/day. For pediatric patients, a dosage level of 2 to 10 mcg manganese/kg/day is recommended.
Aseptic addition of Manganese Sulfate Injection, USP to the TPN solution under a laminar flow hood is recommended. Manganese is physically compatible with the electrolytes and vitamins usually present in the amino acid/dextrose solution used for TPN. Periodic monitoring of manganese plasma levels is suggested as a guideline for subsequent administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
- OVERDOSAGE
-
HOW SUPPLIED
Manganese Sulfate Injection, USP 0.1 mg/mL
NDC: 0517-6410-25 10 mL SDV packed in a box of 25
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
IN6410
Rev. 1/09
MG #14479
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- Container Label
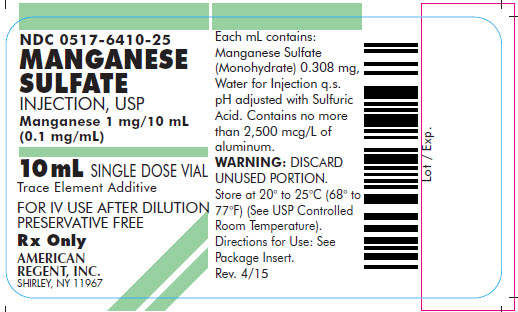
NDC: 0517-6410-25
MANGANESE SULFATE INJECTION, USP
Manganese 1 mg/10 mL
(0.1 mg/mL)
10 mL SINGLE DOSE VIAL
Trace Element Additive
FOR IV USE AFTER DILUTION
PRESERVATIVE FREE
Rx Only
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - Carton Labeling
NDC: 0517-6410-25
25 x 10 mL
SINGLE DOSE VIALS
MANGANESE SULFATE INJECTION, USPManganese 1 mg/10 mL
(0.1 mg/mL)10 mL SINGLE DOSE VIAL
Trace Element Additive
FOR IV USE AFTER DILUTION. PRESERVATIVE FREE
Rx Only
Each mL contains: Manganese Sulfate (Monohydrate) 0.308 mg, Water for Injection q.s.
pH adjusted with Sulfuric Acid. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION. Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature). Directions for Use: See Package Insert.AMERICAN REGENT, INC.
SHIRLEY, NY 11967
Iss. 12/16

- Serialization Label
-
INGREDIENTS AND APPEARANCE
MANGANESE SULFATE
manganese sulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0517-6410 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANGANESE SULFATE (UNII: W00LYS4T26) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE CATION (2+) 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SULFURIC ACID (UNII: O40UQP6WCF) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0517-6410-25 25 in 1 TRAY 09/30/1990 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 09/30/1990 Labeler - American Regent, Inc. (002033710) Establishment Name Address ID/FEI Business Operations American Regent, Inc. 002033710 ANALYSIS(0517-6410) , MANUFACTURE(0517-6410)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
