RANITIDINE - ACID REDUCER- ranitidine hydrochloride tablet, film coated
Ranitidine - Acid Reducer by
Drug Labeling and Warnings
Ranitidine - Acid Reducer by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LLC., Ohm Laboratories Inc., Shasun Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
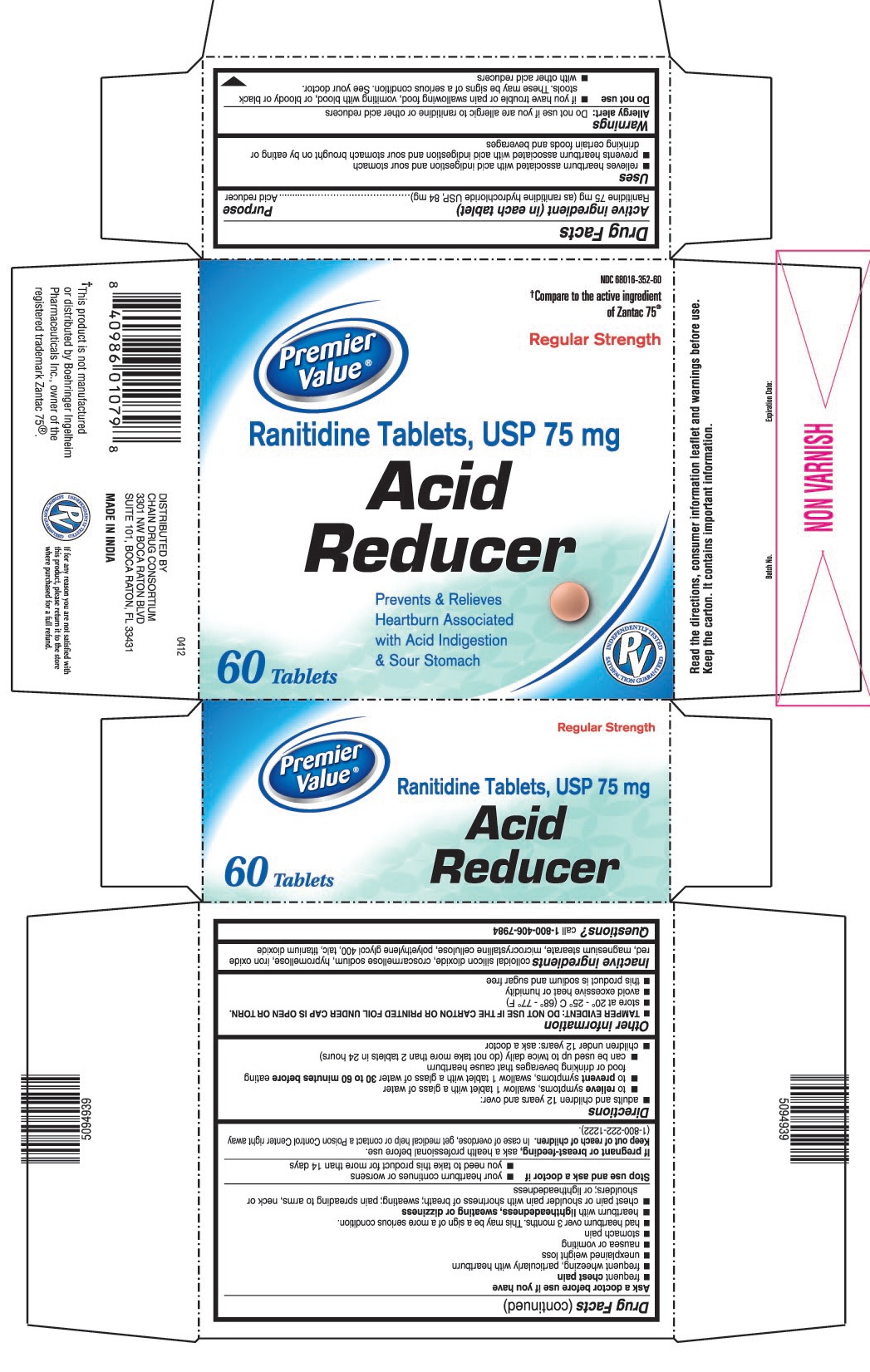
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
-
DIRECTIONS
-
adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
-
adults and children 12 years and over:
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
Premier Value®
NDC: 68016-352-60
Ranitidine Tablets, USP 75 mg
Acid Reducer
Regular Strength
60 Tablets
Prevents & Relieves Heartburn Associated with Acid Indigestion & Sour Stomach
†Compare to the active ingredient of Zantac 75®
†This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals Inc., owner of the registered trademark Zantac 75®.
-
INGREDIENTS AND APPEARANCE
RANITIDINE - ACID REDUCER
ranitidine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-352 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 75 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code OR;606 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-352-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 68016-352-30 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201745 07/10/2012 Labeler - Chain Drug Consortium, LLC. (101668460) Registrant - Ohm Laboratories Inc. (184769029) Establishment Name Address ID/FEI Business Operations Shasun Pharmaceuticals Limited 915786829 MANUFACTURE(68016-352)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.