KEYTRUDA- pembrolizumab injection, powder, lyophilized, for solution KEYTRUDA- pembrolizumab injection, solution
KEYTRUDA by
Drug Labeling and Warnings
KEYTRUDA by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp., MSD International GmbH , MSD Ireland (Carlow), N.V. Organon, Covance Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KEYTRUDA safely and effectively. See full prescribing information for KEYTRUDA.
KEYTRUDA® (pembrolizumab) injection, for intravenous use
Initial U.S. Approval: 2014RECENT MAJOR CHANGES
INDICATIONS AND USAGE
KEYTRUDA is a programmed death receptor-1 (PD-1)-blocking antibody indicated:
Melanoma
- for the treatment of patients with unresectable or metastatic melanoma. (1.1)
- for the adjuvant treatment of patients with melanoma with involvement of lymph node(s) following complete resection. (1.1)
Non-Small Cell Lung Cancer (NSCLC)
- in combination with pemetrexed and platinum chemotherapy, as first-line treatment of patients with metastatic nonsquamous NSCLC, with no EGFR or ALK genomic tumor aberrations. (1.2)
- in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, as first-line treatment of patients with metastatic squamous NSCLC. (1.2)
- as a single agent for the first-line treatment of patients with NSCLC expressing PD-L1 [Tumor Proportion Score (TPS) ≥1%] as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and is:
- as a single agent for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA. (1.2, 2.1)
Small Cell Lung Cancer (SCLC)
- for the treatment of patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.1 (1.3)
Head and Neck Squamous Cell Cancer (HNSCC)
- in combination with platinum and FU for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC. (1.4)
- as a single agent for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test. (1.4, 2.1)
- as a single agent for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy. (1.4)
Classical Hodgkin Lymphoma (cHL)
- for the treatment of adult and pediatric patients with refractory cHL, or who have relapsed after 3 or more prior lines of therapy.1 (1.5)
Primary Mediastinal Large B-Cell Lymphoma (PMBCL)
- for the treatment of adult and pediatric patients with refractory PMBCL, or who have relapsed after 2 or more prior lines of therapy.1 (1.6)
- Limitations of Use: KEYTRUDA is not recommended for treatment of patients with PMBCL who require urgent cytoreductive therapy.
Urothelial Carcinoma
- for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 [Combined Positive Score (CPS) ≥10] as determined by an FDA-approved test, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status.1 (1.7, 2.1)
- for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. (1.7)
- for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. (1.7)
Microsatellite Instability-High Cancer
- for the treatment of adult and pediatric patients with unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient
- solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options,1 or
- colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.1 (1.8)
- Limitations of Use: The safety and effectiveness of KEYTRUDA in pediatric patients with MSI-H central nervous system cancers have not been established. (1.8)
Gastric Cancer
- for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test, with disease progression on or after 2 or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.1 (1.9, 2.1)
Esophageal Cancer
- for the treatment of patients with recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus whose tumors express PD-L1 [Combined Positive Score (CPS) ≥10] as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy. (1.10, 2.1)
Cervical Cancer
- for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test.1 (1.11, 2.1)
Hepatocellular Carcinoma (HCC)
- for the treatment of patients with HCC who have been previously treated with sorafenib.1 (1.12)
Merkel Cell Carcinoma (MCC)
- for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma.1 (1.13)
Renal Cell Carcinoma (RCC)
- in combination with axitinib, for the first-line treatment of patients with advanced RCC. (1.14)
Endometrial Carcinoma
- in combination with lenvatinib, for the treatment of patients with advanced endometrial carcinoma that is not MSI-H or dMMR, who have disease progression following prior systemic therapy and are not candidates for curative surgery or radiation.1 (1.15)
1 This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
DOSAGE AND ADMINISTRATION
- Melanoma: 200 mg every 3 weeks. (2.2)
- NSCLC: 200 mg every 3 weeks. (2.3)
- SCLC: 200 mg every 3 weeks (2.4)
- HNSCC: 200 mg every 3 weeks. (2.5)
- cHL or PMBCL: 200 mg every 3 weeks for adults; 2 mg/kg (up to 200 mg) every 3 weeks for pediatrics. (2.6, 2.7)
- Urothelial Carcinoma: 200 mg every 3 weeks. (2.8)
- MSI-H Cancer: 200 mg every 3 weeks for adults and 2 mg/kg (up to 200 mg) every 3 weeks for pediatrics. (2.9)
- Gastric Cancer: 200 mg every 3 weeks. (2.10)
- Esophageal Cancer: 200 mg every 3 weeks. (2.11)
- Cervical Cancer: 200 mg every 3 weeks. (2.12)
- HCC: 200 mg every 3 weeks. (2.13)
- MCC: 200 mg every 3 weeks for adults; 2 mg/kg (up to 200 mg) every 3 weeks for pediatrics. (2.14)
- RCC: 200 mg every 3 weeks with axitinib 5 mg orally twice daily. (2.15)
- Endometrial Carcinoma: 200 mg every 3 weeks with lenvatinib 20 mg orally once daily for tumors that are not MSI-H or dMMR. (2.16)
Administer KEYTRUDA as an intravenous infusion over 30 minutes.
DOSAGE FORMS AND STRENGTHS
- Injection: 100 mg/4 mL (25 mg/mL) solution in a single-dose vial (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Immune-mediated pneumonitis: Withhold for moderate, and permanently discontinue for severe, life-threatening or recurrent moderate pneumonitis. (5.1)
- Immune-mediated colitis: Withhold for moderate or severe, and permanently discontinue for life-threatening colitis. (5.2)
- Immune-mediated hepatitis (KEYTRUDA) and hepatotoxicity (KEYTRUDA in combination with axitinib): Monitor for changes in hepatic function. Based on severity of liver enzyme elevations, withhold or discontinue KEYTRUDA, axitinib, or KEYTRUDA and axitinib. Consider corticosteroid therapy. (2.17, 5.3)
- Immune-mediated endocrinopathies (5.4):
- Adrenal insufficiency: Withhold for moderate and withhold or permanently discontinue for severe or life-threatening adrenal insufficiency.
- Hypophysitis: Withhold for moderate and withhold or permanently discontinue for severe or life-threatening hypophysitis.
- Thyroid disorders: Monitor for changes in thyroid function. Withhold or permanently discontinue for severe or life-threatening hyperthyroidism.
- Type 1 diabetes mellitus: Monitor for hyperglycemia. Withhold KEYTRUDA in cases of severe hyperglycemia.
- Immune-mediated nephritis: Monitor for changes in renal function. Withhold for moderate, and permanently discontinue for severe or life-threatening nephritis. (5.5)
- Immune-mediated skin adverse reactions including, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN): Withhold for severe and permanently discontinue for life-threatening skin reactions. (5.6)
- Other immune-mediated adverse reactions: In organ transplant recipients, consider the benefit of treatment with KEYTRUDA versus the risk of possible organ rejection. (5.7)
- Infusion-related reactions: Stop infusion and permanently discontinue KEYTRUDA for severe or life-threatening infusion reactions. (5.8)
- Complications of allogeneic HSCT (5.9):
- Allogeneic HSCT after treatment with KEYTRUDA: Monitor for hepatic veno-occlusive disease, grade 3-4 acute GVHD including hyperacute GVHD, steroid-requiring febrile syndrome, and other immune-mediated adverse reactions. Transplant-related mortality has occurred.
- Allogeneic HSCT prior to treatment with KEYTRUDA: In patients with a history of allogeneic HSCT, consider the benefit of treatment with KEYTRUDA versus the risk of GVHD.
- Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials. (5.10)
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective method of contraception. (5.11, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (reported in ≥20% of patients) were:
- KEYTRUDA as a single agent: fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. (6.1)
- KEYTRUDA in combination with chemotherapy: fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, pyrexia, alopecia, peripheral neuropathy, mucosal inflammation, and stomatitis. (6.1)
- KEYTRUDA in combination with axitinib: diarrhea, fatigue/asthenia, hypertension, hepatotoxicity, hypothyroidism, decreased appetite, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation. (6.1)
- KEYTRUDA in combination with lenvatinib: fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Melanoma
1.2 Non-Small Cell Lung Cancer
1.3 Small Cell Lung Cancer
1.4 Head and Neck Squamous Cell Cancer
1.5 Classical Hodgkin Lymphoma
1.6 Primary Mediastinal Large B-Cell Lymphoma
1.7 Urothelial Carcinoma
1.8 Microsatellite Instability-High Cancer
1.9 Gastric Cancer
1.10 Esophageal Cancer
1.11 Cervical Cancer
1.12 Hepatocellular Carcinoma
1.13 Merkel Cell Carcinoma
1.14 Renal Cell Carcinoma
1.15 Endometrial Carcinoma
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for NSCLC, HNSCC, Urothelial Carcinoma, Gastric Cancer, Esophageal Cancer, or Cervical Cancer
2.2 Recommended Dosage for Melanoma
2.3 Recommended Dosage for NSCLC
2.4 Recommended Dosage for SCLC
2.5 Recommended Dosage for HNSCC
2.6 Recommended Dosage for cHL
2.7 Recommended Dosage for PMBCL
2.8 Recommended Dosage for Urothelial Carcinoma
2.9 Recommended Dosage for MSI-H Cancer
2.10 Recommended Dosage for Gastric Cancer
2.11 Recommended Dosage for Esophageal Cancer
2.12 Recommended Dosage for Cervical Cancer
2.13 Recommended Dosage for HCC
2.14 Recommended Dosage for MCC
2.15 Recommended Dosage for RCC
2.16 Recommended Dosage for Endometrial Carcinoma
2.17 Dose Modifications
2.18 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Immune-Mediated Pneumonitis
5.2 Immune-Mediated Colitis
5.3 Immune-Mediated Hepatitis (KEYTRUDA) and Hepatotoxicity (KEYTRUDA in Combination with Axitinib)
5.4 Immune-Mediated Endocrinopathies
5.5 Immune-Mediated Nephritis and Renal Dysfunction
5.6 Immune-Mediated Skin Adverse Reactions
5.7 Other Immune-Mediated Adverse Reactions
5.8 Infusion-Related Reactions
5.9 Complications of Allogeneic HSCT
5.10 Increased Mortality in Patients with Multiple Myeloma when KEYTRUDA is Added to a Thalidomide Analogue and Dexamethasone
5.11 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Melanoma
14.2 Non-Small Cell Lung Cancer
14.3 Small Cell Lung Cancer
14.4 Head and Neck Squamous Cell Cancer
14.5 Classical Hodgkin Lymphoma
14.6 Primary Mediastinal Large B-Cell Lymphoma
14.7 Urothelial Carcinoma
14.8 Microsatellite Instability-High Cancer
14.9 Gastric Cancer
14.10 Esophageal Cancer
14.11 Cervical Cancer
14.12 Hepatocellular Carcinoma
14.13 Merkel Cell Carcinoma
14.14 Renal Cell Carcinoma
14.15 Endometrial Carcinoma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Melanoma
KEYTRUDA® (pembrolizumab) is indicated for the treatment of patients with unresectable or metastatic melanoma.
KEYTRUDA is indicated for the adjuvant treatment of patients with melanoma with involvement of lymph node(s) following complete resection.
1.2 Non-Small Cell Lung Cancer
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
KEYTRUDA, in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, is indicated for the first-line treatment of patients with metastatic squamous NSCLC.
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with NSCLC expressing PD-L1 [Tumor Proportion Score (TPS) ≥1%] as determined by an FDA-approved test [see Dosage and Administration (2.1)], with no EGFR or ALK genomic tumor aberrations, and is:
- stage III where patients are not candidates for surgical resection or definitive chemoradiation, or
- metastatic.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test [see Dosage and Administration (2.1)], with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA.
1.3 Small Cell Lung Cancer
KEYTRUDA is indicated for the treatment of patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.3)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
1.4 Head and Neck Squamous Cell Cancer
KEYTRUDA, in combination with platinum and fluorouracil (FU), is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC).
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test [see Dosage and Administration (2.1)].
KEYTRUDA, as a single agent, is indicated for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.
1.5 Classical Hodgkin Lymphoma
KEYTRUDA is indicated for the treatment of adult and pediatric patients with refractory classical Hodgkin lymphoma (cHL), or who have relapsed after 3 or more prior lines of therapy.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.5)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.6 Primary Mediastinal Large B-Cell Lymphoma
KEYTRUDA is indicated for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after 2 or more prior lines of therapy.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.6)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitations of Use: KEYTRUDA is not recommended for treatment of patients with PMBCL who require urgent cytoreductive therapy.
1.7 Urothelial Carcinoma
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-approved test [see Dosage and Administration (2.1)], or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status.
This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies (14.7)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
KEYTRUDA is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
1.8 Microsatellite Instability-High Cancer
KEYTRUDA is indicated for the treatment of adult and pediatric patients with unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR)
- solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options, or
- colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.8)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Limitations of Use: The safety and effectiveness of KEYTRUDA in pediatric patients with MSI-H central nervous system cancers have not been established.
1.9 Gastric Cancer
KEYTRUDA is indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test [see Dosage and Administration (2.1)], with disease progression on or after 2 or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.9)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.10 Esophageal Cancer
KEYTRUDA is indicated for the treatment of patients with recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-approved test [see Dosage and Administration (2.1)], with disease progression after one or more prior lines of systemic therapy.
1.11 Cervical Cancer
KEYTRUDA is indicated for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test [see Dosage and Administration (2.1)].
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.11)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.12 Hepatocellular Carcinoma
KEYTRUDA is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.12)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.13 Merkel Cell Carcinoma
KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.13)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.14 Renal Cell Carcinoma
KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of patients with advanced renal cell carcinoma (RCC).
1.15 Endometrial Carcinoma
KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of patients with advanced endometrial carcinoma that is not MSI-H or dMMR, who have disease progression following prior systemic therapy and are not candidates for curative surgery or radiation.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.15)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for NSCLC, HNSCC, Urothelial Carcinoma, Gastric Cancer, Esophageal Cancer, or Cervical Cancer
Select patients for treatment with KEYTRUDA as a single agent based on the presence of positive PD-L1 expression in:
- stage III NSCLC who are not candidates for surgical resection or definitive chemoradiation [see Clinical Studies (14.2)].
- metastatic NSCLC [see Clinical Studies (14.2)].
- first-line treatment of metastatic or unresectable, recurrent HNSCC [see Clinical Studies (14.4)].
- metastatic urothelial carcinoma [see Clinical Studies (14.7)].
- metastatic gastric cancer [see Clinical Studies (14.9)]. If PD-L1 expression is not detected in an archival gastric cancer specimen, evaluate the feasibility of obtaining a tumor biopsy for PD-L1 testing.
- metastatic esophageal cancer [see Clinical Studies (14.10)].
- recurrent or metastatic cervical cancer [see Clinical Studies (14.11)].
Information on FDA-approved tests for the detection of PD-L1 expression for these indications is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage for Melanoma
The recommended dose of KEYTRUDA in patients with unresectable or metastatic melanoma is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity.
The recommended dose of KEYTRUDA for the adjuvant treatment of adult patients with melanoma is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease recurrence, unacceptable toxicity, or for up to 12 months in patients without disease recurrence.
2.3 Recommended Dosage for NSCLC
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
When administering KEYTRUDA in combination with chemotherapy, administer KEYTRUDA prior to chemotherapy when given on the same day. Refer to the Prescribing Information for the chemotherapy agents administered in combination with KEYTRUDA for recommended dosing information, as appropriate.
2.4 Recommended Dosage for SCLC
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
2.5 Recommended Dosage for HNSCC
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
When administering KEYTRUDA in combination with chemotherapy, administer KEYTRUDA prior to chemotherapy when given on the same day. Refer to the Prescribing Information for the chemotherapy agents administered in combination with KEYTRUDA for recommended dosing information, as appropriate.
2.6 Recommended Dosage for cHL
The recommended dose of KEYTRUDA in adults is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
The recommended dose of KEYTRUDA in pediatric patients is 2 mg/kg (up to a maximum of 200 mg), administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
2.7 Recommended Dosage for PMBCL
The recommended dose of KEYTRUDA in adults is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
The recommended dose of KEYTRUDA in pediatric patients is 2 mg/kg (up to a maximum of 200 mg), administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
2.8 Recommended Dosage for Urothelial Carcinoma
The recommended dose of KEYTRUDA in patients with locally advanced or metastatic urothelial carcinoma is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
The recommended dose of KEYTRUDA in patients with high-risk BCG-unresponsive non-muscle invasive bladder cancer is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until persistent or recurrent high-risk NMIBC, disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
2.9 Recommended Dosage for MSI-H Cancer
The recommended dose of KEYTRUDA in adults is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
The recommended dose of KEYTRUDA in pediatric patients is 2 mg/kg (up to a maximum of 200 mg), administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
2.10 Recommended Dosage for Gastric Cancer
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
2.11 Recommended Dosage for Esophageal Cancer
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
2.12 Recommended Dosage for Cervical Cancer
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
2.13 Recommended Dosage for HCC
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
2.14 Recommended Dosage for MCC
The recommended dose of KEYTRUDA in adults is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
The recommended dose of KEYTRUDA in pediatric patients is 2 mg/kg (up to a maximum of 200 mg), administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
2.15 Recommended Dosage for RCC
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks in combination with 5 mg axitinib orally twice daily until disease progression, unacceptable toxicity, or for KEYTRUDA, up to 24 months in patients without disease progression. When axitinib is used in combination with KEYTRUDA, dose escalation of axitinib above the initial 5 mg dose may be considered at intervals of six weeks or longer. See also the Prescribing Information for recommended axitinib dosing information.
2.16 Recommended Dosage for Endometrial Carcinoma
The recommended dose of KEYTRUDA is 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks in combination with lenvatinib 20 mg orally once daily until disease progression, unacceptable toxicity, or for KEYTRUDA, up to 24 months in patients without disease progression.
Refer to the lenvatinib prescribing information for recommended dosing information.
2.17 Dose Modifications
No dose reductions of KEYTRUDA are recommended. Withhold or discontinue KEYTRUDA to manage adverse reactions as described in Table 1.
Table 1: Recommended Dose Modifications for Adverse Reactions [see Warnings and Precautions (5.1-5.9)] Adverse Reaction Severity* Dose Modification for KEYTRUDA - * Toxicity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events. Version 4.0 (NCI CTCAE v4)
- † Resume in patients with complete or partial resolution (Grades 0 to 1) after corticosteroid taper.
- ‡ Resume in HCC patients when AST or ALT and total bilirubin recover to Grades 0-1 or to baseline.
Immune-mediated pneumonitis Grade 2 Withhold† Grades 3 or 4 or recurrent Grade 2 Permanently discontinue Immune-mediated colitis Grades 2 or 3 Withhold† Grade 4 Permanently discontinue Immune-mediated hepatitis in patients with HCC Aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than or equal to 5 times upper limit of normal (ULN) if baseline less than 2 times ULN;
AST or ALT greater than 3 times baseline if baseline greater than or equal to 2 times ULN
Total bilirubin greater than 2.0 mg/dL if baseline less than 1.5 mg/dL; or
Total bilirubin greater than 3.0 mg/dL, regardless of baseline levelsWithhold‡ ALT or AST greater than 10 times ULN; or Child-Pugh score greater than or equal to 9 points;
Gastrointestinal bleeding suggestive of portal hypertension; or
New onset of clinically detectable ascites; or encephalopathyPermanently discontinue Immune-mediated hepatitis in patients without HCC AST or ALT greater than 3 but no more than 5 times the ULN or total bilirubin greater than 1.5 but no more than 3 times the ULN Withhold† For liver enzyme elevations in RCC patients treated with combination therapy, see dosing guidelines following this table. In patients without liver metastases, AST or ALT greater than 5 times ULN or total bilirubin greater than 3 times ULN
In patients with liver metastasis and Grade 2 AST or ALT at baseline, with an increase in AST or ALT of 50% or more relative to baseline that persists for at least 1 weekPermanently discontinue Immune-mediated endocrinopathies Grades 3 or 4 Withhold until clinically stable Immune-mediated nephritis Grade 2 Withhold† Grades 3 or 4 Permanently discontinue Immune-mediated skin adverse reactions Grade 3 or suspected Stevens-Johnson Syndrome (SJS) or toxic epidermal necrolysis (TEN) Withhold Grade 4 or confirmed SJS or TEN Permanently discontinue Hematologic toxicity in patients with cHL or PMBCL Grade 4 Withhold until resolution to Grades 0 or 1 Other immune-mediated adverse reactions Grades 2 or 3 based on the severity and type of reaction Withhold† Grade 3 based on the severity and type of reaction or Grade 4 Permanently discontinue Recurrent immune-mediated adverse reactions Recurrent Grade 2 pneumonitis
Recurrent Grades 3 or 4Permanently discontinue Inability to taper corticosteroid Requirement for 10 mg per day or greater prednisone or equivalent for more than 12 weeks after last dose of KEYTRUDA Permanently discontinue Persistent Grade 2 or 3 adverse reaction (excluding endocrinopathy) Grades 2 or 3 adverse reactions lasting 12 weeks or longer after last dose of KEYTRUDA Permanently discontinue Infusion-related reactions Grades 1 or 2 Interrupt or slow the rate of infusion Grades 3 or 4 Permanently discontinue In patients with RCC being treated with KEYTRUDA in combination with axitinib:
- If ALT or AST ≥3 times ULN but <10 times ULN without concurrent total bilirubin ≥2 times ULN, withhold both KEYTRUDA and axitinib until these adverse reactions recover to Grades 0-1. Consider corticosteroid therapy. Consider rechallenge with a single drug or sequential rechallenge with both drugs after recovery. If rechallenging with axitinib, consider dose reduction as per the axitinib Prescribing Information.
- If ALT or AST ≥10 times ULN or >3 times ULN with concurrent total bilirubin ≥2 times ULN, permanently discontinue both KEYTRUDA and axitinib and consider corticosteroid therapy.
When administering KEYTRUDA in combination with lenvatinib for the treatment of endometrial carcinoma, interrupt one or both as appropriate. No dose reductions are recommended for KEYTRUDA. Withhold, dose reduce, or discontinue lenvatinib in accordance with the instructions in the lenvatinib prescribing information.
2.18 Preparation and Administration
Preparation for Intravenous Infusion
- Visually inspect the solution for particulate matter and discoloration. The solution is clear to slightly opalescent, colorless to slightly yellow. Discard the vial if visible particles are observed.
- Dilute KEYTRUDA injection (solution) prior to intravenous administration.
- Withdraw the required volume from the vial(s) of KEYTRUDA and transfer into an intravenous (IV) bag containing 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Mix diluted solution by gentle inversion. Do not shake. The final concentration of the diluted solution should be between 1 mg/mL to 10 mg/mL.
- Discard any unused portion left in the vial.
Storage of Diluted Solution
The product does not contain a preservative.
Store the diluted solution from the KEYTRUDA 100 mg/4 mL vial either:
- At room temperature for no more than 6 hours from the time of dilution. This includes room temperature storage of the diluted solution, and the duration of infusion.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 96 hours from the time of dilution. If refrigerated, allow the diluted solution to come to room temperature prior to administration. Do not shake.
Discard after 6 hours at room temperature or after 96 hours under refrigeration.
Do not freeze.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Immune-Mediated Pneumonitis
KEYTRUDA can cause immune-mediated pneumonitis, including fatal cases. Monitor patients for signs and symptoms of pneumonitis. Evaluate patients with suspected pneumonitis with radiographic imaging and administer corticosteroids (initial dose of 1 to 2 mg/kg/day prednisone or equivalent followed by a taper) for Grade 2 or greater pneumonitis. Withhold KEYTRUDA for moderate (Grade 2) pneumonitis, and permanently discontinue KEYTRUDA for severe (Grade 3), life-threatening (Grade 4), or recurrent moderate (Grade 2) pneumonitis [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
In clinical studies enrolling 2799 patients with various cancers who received KEYTRUDA as a single agent, pneumonitis occurred in 94 (3.4%) patients, including Grade 1 (0.8%), Grade 2 (1.3%), Grade 3 (0.9%), Grade 4 (0.3%), and Grade 5 (0.1%) pneumonitis. The median time to onset was 3.3 months (range: 2 days to 19.3 months), and the median duration was 1.5 months (range: 1 day to 17.2+ months). Sixty-three (67%) of the 94 patients received systemic corticosteroids, with 50 of the 63 receiving high-dose corticosteroids for a median duration of 8 days (range: 1 day to 10.1 months) followed by a corticosteroid taper. Pneumonitis occurred more frequently in patients with a history of prior thoracic radiation (6.9%) than in patients who did not receive prior thoracic radiation (2.9%). Pneumonitis led to discontinuation of KEYTRUDA in 36 (1.3%) patients. Pneumonitis resolved in 55 (59%) of the 94 patients.
In clinical studies enrolling 790 patients with NSCLC who received KEYTRUDA as a single agent as first-line therapy for advanced disease, pneumonitis occurred in 65 (8.2%) patients, including Grades 3-4 in 3.2% of patients. Forty-eight of the 65 patients received high-dose corticosteroids for a median duration of 5 days (range: 1 to 26 days). Pneumonitis occurred in 17% of patients with a history of prior thoracic radiation and 7.7% of patients who did not receive prior thoracic radiation. Pneumonitis led to discontinuation of KEYTRUDA in 29 (3.7%) patients. Pneumonitis resolved in 51% of the patients.
In KEYNOTE-048 enrolling 300 patients with HNSCC who received KEYTRUDA as a single agent pneumonitis occurred in 18 (6%) patients, including Grade 3 (1.3%), Grade 4 (0%), and Grade 5 (0.3%). Eight of the 18 patients received high-dose corticosteroids for a median duration of 14 days (range: 1 to 77 days). Pneumonitis led to discontinuation of KEYTRUDA in 2 (0.7%) patients. Pneumonitis resolved in 12 (66%) of the patients. Pneumonitis occurred in 15 (5.4%) patients of 276 patients with HNSCC receiving KEYTRUDA in combination with platinum and FU as first-line therapy for advanced disease, including Grade 3 (1.1%), Grade 4 (0%), and Grade 5 (0.4%) pneumonitis. Four of the 15 patients received high-dose corticosteroids for a median duration of 16 days (range: 2 to 32 days). Pneumonitis led to discontinuation of KEYTRUDA in 5 (1.8%) patients. Pneumonitis resolved in 12 (80%) of the patients.
5.2 Immune-Mediated Colitis
KEYTRUDA can cause immune-mediated colitis. Monitor patients for signs and symptoms of colitis. Administer corticosteroids (initial dose of 1 to 2 mg/kg/day prednisone or equivalent followed by a taper) for Grade 2 or greater colitis. Withhold KEYTRUDA for moderate (Grade 2) or severe (Grade 3) colitis, and permanently discontinue KEYTRUDA for life-threatening (Grade 4) colitis [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Colitis occurred in 48 (1.7%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.4%), Grade 3 (1.1%), and Grade 4 (<0.1%) colitis. The median time to onset was 3.5 months (range: 10 days to 16.2 months), and the median duration was 1.3 months (range: 1 day to 8.7+ months). Thirty-three (69%) of the 48 patients received systemic corticosteroids, with 27 of the 33 requiring high-dose corticosteroids for a median duration of 7 days (range: 1 day to 5.3 months) followed by a corticosteroid taper. Colitis led to discontinuation of KEYTRUDA in 15 (0.5%) patients. Colitis resolved in 41 (85%) of the 48 patients.
5.3 Immune-Mediated Hepatitis (KEYTRUDA) and Hepatotoxicity (KEYTRUDA in Combination with Axitinib)
Immune-Mediated Hepatitis
KEYTRUDA can cause immune-mediated hepatitis. Monitor patients for changes in liver function. Administer corticosteroids (initial dose of 0.5 to 1 mg/kg/day [for Grade 2 hepatitis] and 1 to 2 mg/kg/day [for Grade 3 or greater hepatitis] prednisone or equivalent followed by a taper) and, based on severity of liver enzyme elevations, withhold or discontinue KEYTRUDA [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Hepatitis occurred in 19 (0.7%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.1%), Grade 3 (0.4%), and Grade 4 (<0.1%) hepatitis. The median time to onset was 1.3 months (range: 8 days to 21.4 months), and the median duration was 1.8 months (range: 8 days to 20.9+ months). Thirteen (68%) of the 19 patients received systemic corticosteroids, with 12 of the 13 receiving high-dose corticosteroids for a median duration of 5 days (range: 1 to 26 days) followed by a corticosteroid taper. Hepatitis led to discontinuation of KEYTRUDA in 6 (0.2%) patients. Hepatitis resolved in 15 (79%) of the 19 patients.
Hepatotoxicity in Combination with Axitinib
KEYTRUDA in combination with axitinib can cause hepatic toxicity with higher than expected frequencies of Grades 3 and 4 ALT and AST elevations compared to KEYTRUDA alone. Monitor liver enzymes before initiation of and periodically throughout treatment. Consider more frequent monitoring of liver enzymes as compared to when the drugs are administered as single agents. For elevated liver enzymes, interrupt KEYTRUDA and axitinib and consider administering corticosteroids as needed [see Dosage and Administration (2.17)].
With the combination of KEYTRUDA and axitinib, Grades 3 and 4 increased ALT (20%) and increased AST (13%) were seen. The median time to onset of increased ALT was 2.3 months (range: 7 days to 19.8 months). Fifty-nine percent of the patients with increased ALT received systemic corticosteroids. In patients with ALT ≥3 times ULN (Grades 2-4, n=116), ALT resolved to Grades 0-1 in 94%. Among the 92 patients who were rechallenged with either KEYTRUDA (3%) or axitinib (31%) administered as a single agent or with both (50%), 55% had no recurrence of ALT >3 times ULN.
5.4 Immune-Mediated Endocrinopathies
Adrenal Insufficiency
KEYTRUDA can cause adrenal insufficiency (primary and secondary). Monitor for signs and symptoms of adrenal insufficiency. Administer corticosteroids and hormone replacement as clinically indicated. Withhold KEYTRUDA for moderate (Grade 2) adrenal insufficiency and withhold or discontinue KEYTRUDA for severe (Grade 3) or life-threatening (Grade 4) adrenal insufficiency [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Adrenal insufficiency occurred in 0.8% (22/2799) of patients receiving KEYTRUDA, including Grade 4 (<0.1%), Grade 3 (0.3%), and Grade 2 (0.3%) adrenal insufficiency. The median time to onset was 5.3 months (range: 26 days to 16.6 months), and the median duration was not reached (range: 4 days to 1.9+ years). Adrenal insufficiency led to permanent discontinuation of KEYTRUDA in <0.1% of patients and withholding of KEYTRUDA in 0.3% of patients.
Systemic corticosteroids were required in 77% (17/22) of patients with adrenal insufficiency, including 9% who received high-dose corticosteroids (prednisone ≥40 mg per day or equivalent) for median duration of 4 days (range: 1 to 6 days) followed by corticosteroid taper. Adrenal insufficiency resolved in 23% of the patients.
Hypophysitis
KEYTRUDA can cause hypophysitis. Monitor for signs and symptoms of hypophysitis (including hypopituitarism). Administer corticosteroids and hormone replacement as clinically indicated. Withhold KEYTRUDA for moderate (Grade 2) hypophysitis and withhold or discontinue KEYTRUDA for severe (Grade 3) or life-threatening (Grade 4) hypophysitis [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Hypophysitis occurred in 17 (0.6%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.2%), Grade 3 (0.3%), and Grade 4 (<0.1%) hypophysitis. The median time to onset was 3.7 months (range: 1 day to 11.9 months), and the median duration was 4.7 months (range: 8+ days to 12.7+ months). Sixteen (94%) of the 17 patients received systemic corticosteroids, with 6 of the 16 receiving high-dose corticosteroids. Hypophysitis led to discontinuation of KEYTRUDA in 4 (0.1%) patients. Hypophysitis resolved in 7 (41%) of the 17 patients.
Thyroid Disorders
KEYTRUDA can cause thyroid disorders, including hyperthyroidism, hypothyroidism and thyroiditis. Monitor patients for changes in thyroid function (at the start of treatment, periodically during treatment, and as indicated based on clinical evaluation) and for clinical signs and symptoms of thyroid disorders. Administer replacement hormones for hypothyroidism and manage hyperthyroidism with thionamides and beta-blockers as appropriate. Withhold or discontinue KEYTRUDA for severe (Grade 3) or life-threatening (Grade 4) hyperthyroidism [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Hyperthyroidism occurred in 96 (3.4%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.8%) and Grade 3 (0.1%) hyperthyroidism. The median time to onset was 1.4 months (range: 1 day to 21.9 months), and the median duration was 2.1 months (range: 3 days to 15.0+ months). Hyperthyroidism led to discontinuation of KEYTRUDA in 2 (<0.1%) patients. Hyperthyroidism resolved in 71 (74%) of the 96 patients.
Hypothyroidism occurred in 237 (8.5%) of 2799 patients receiving KEYTRUDA, including Grade 2 (6.2%) and Grade 3 (0.1%) hypothyroidism. The median time to onset was 3.5 months (range: 1 day to 18.9 months), and the median duration was not reached (range: 2 days to 27.7+ months). Hypothyroidism led to discontinuation of KEYTRUDA in 1 (<0.1%) patient. Hypothyroidism resolved in 48 (20%) of the 237 patients. The incidence of new or worsening hypothyroidism was higher in 1185 patients with HNSCC (16%) receiving KEYTRUDA as a single agent or in combination with platinum and FU, including Grade 3 (0.3%) hypothyroidism.
Thyroiditis occurred in 16 (0.6%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.3%) thyroiditis. The median time of onset was 1.2 months (range: 0.5 to 3.5 months).
Type 1 Diabetes mellitus
KEYTRUDA can cause type 1 diabetes mellitus, including diabetic ketoacidosis, which have been reported in 6 (0.2%) of 2799 patients receiving KEYTRUDA. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Administer insulin for type 1 diabetes and withhold KEYTRUDA and administer anti-hyperglycemics in patients with severe hyperglycemia [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
5.5 Immune-Mediated Nephritis and Renal Dysfunction
KEYTRUDA can cause immune-mediated nephritis. Monitor patients for changes in renal function. Administer corticosteroids (initial dose of 1 to 2 mg/kg/day prednisone or equivalent followed by a taper) for Grade 2 or greater nephritis. Withhold KEYTRUDA for moderate (Grade 2), and permanently discontinue KEYTRUDA for severe (Grade 3) or life-threatening (Grade 4) nephritis [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
Nephritis occurred in 9 (0.3%) of 2799 patients receiving KEYTRUDA, including Grade 2 (0.1%), Grade 3 (0.1%), and Grade 4 (<0.1%) nephritis. The median time to onset was 5.1 months (range: 12 days to 12.8 months), and the median duration was 3.3 months (range: 12 days to 8.9+ months). Eight (89%) of the 9 patients received systemic corticosteroids, with 7 of the 8 receiving high-dose corticosteroids for a median duration of 15 days (range: 3 days to 4.0 months) followed by a corticosteroid taper. Nephritis led to discontinuation of KEYTRUDA in 3 (0.1%) patients. Nephritis resolved in 5 (56%) of the 9 patients. Nephritis occurred in 1.7% of 405 patients receiving KEYTRUDA in combination with pemetrexed and platinum in the KEYNOTE-189 study, including Grade 3 (1%) and Grade 4 (0.5%) nephritis. The median time to onset was 3.2 months (range: 16 days to 11.1 months) and the duration ranged from 1.6 to 16.8+ months. Six (86%) of the 7 patients received systemic corticosteroids, with all 6 receiving high-dose corticosteroids for a median duration of 3 days (range: 1 to 17 days) followed by a corticosteroid taper. Nephritis led to discontinuation of KEYTRUDA in 5 (1.2%) patients. Nephritis resolved in 2 (29%) of the 7 patients.
5.6 Immune-Mediated Skin Adverse Reactions
Immune-mediated rashes, including SJS, TEN (some cases with fatal outcome), exfoliative dermatitis, and bullous pemphigoid, can occur. Monitor patients for suspected severe skin reactions and exclude other causes. Based on the severity of the adverse reaction, withhold or permanently discontinue KEYTRUDA and administer corticosteroids. For signs or symptoms of SJS or TEN, withhold KEYTRUDA and refer the patient for specialized care for assessment and treatment. If SJS or TEN is confirmed, permanently discontinue KEYTRUDA [see Dosage and Administration (2.17)].
5.7 Other Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue in patients receiving KEYTRUDA. While immune-mediated adverse reactions usually occur during treatment with PD-1/PD-L1 blocking antibodies, they may occur after discontinuation of treatment.
For suspected immune-mediated adverse reactions, ensure adequate evaluation to confirm etiology or exclude other causes. Based on the severity of the adverse reaction, withhold KEYTRUDA and administer corticosteroids. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Based on limited data from clinical studies in patients whose immune-related adverse reactions could not be controlled with corticosteroid use, administration of other systemic immunosuppressants can be considered. Resume KEYTRUDA when the immune-mediated adverse reaction remains at Grade 1 or less following corticosteroid taper. Permanently discontinue KEYTRUDA for any Grade 3 immune-mediated adverse reaction that recurs and for any life-threatening immune-mediated adverse reaction [see Dosage and Administration (2.17) and Adverse Reactions (6.1)].
The following clinically significant, immune-mediated adverse reactions occurred in less than 1% (unless otherwise indicated) of 2799 patients treated with KEYTRUDA: arthritis (1.5%), uveitis, myositis, Guillain-Barré syndrome, myasthenia gravis, vasculitis, pancreatitis, hemolytic anemia, sarcoidosis, and encephalitis. In addition, myelitis and myocarditis were reported in other trials, including cHL, and post-marketing use.
Solid organ transplant rejection has been reported in the post-marketing setting in patients treated with KEYTRUDA. Treatment with KEYTRUDA may increase the risk of rejection in solid organ transplant recipients. Consider the benefit of treatment with KEYTRUDA versus the risk of possible organ rejection in these patients.
5.8 Infusion-Related Reactions
KEYTRUDA can cause severe or life-threatening infusion-related reactions, including hypersensitivity and anaphylaxis, which have been reported in 6 (0.2%) of 2799 patients receiving KEYTRUDA. Monitor patients for signs and symptoms of infusion-related reactions including rigors, chills, wheezing, pruritus, flushing, rash, hypotension, hypoxemia, and fever. For severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions, stop infusion and permanently discontinue KEYTRUDA [see Dosage and Administration (2.17)].
5.9 Complications of Allogeneic HSCT
Allogeneic HSCT after treatment with KEYTRUDA
Immune-mediated complications, including fatal events, occurred in patients who underwent allogeneic hematopoietic stem cell transplantation (HSCT) after being treated with KEYTRUDA. Of 23 patients with cHL who proceeded to allogeneic HSCT after treatment with KEYTRUDA on any trial, 6 patients (26%) developed graft-versus-host-disease (GVHD), one of which was fatal, and 2 patients (9%) developed severe hepatic veno-occlusive disease (VOD) after reduced-intensity conditioning, one of which was fatal. Cases of fatal hyperacute GVHD after allogeneic HSCT have also been reported in patients with lymphoma who received a PD-1 receptor blocking antibody before transplantation. These complications may occur despite intervening therapy between PD-1 blockade and allogeneic HSCT. Follow patients closely for early evidence of transplant-related complications such as hyperacute GVHD, severe (Grade 3 to 4) acute GVHD, steroid-requiring febrile syndrome, hepatic VOD, and other immune-mediated adverse reactions, and intervene promptly.
Allogeneic HSCT prior to treatment with KEYTRUDA
In patients with a history of allogeneic HSCT, acute GVHD, including fatal GVHD, has been reported after treatment with KEYTRUDA. Patients who experienced GVHD after their transplant procedure may be at increased risk for GVHD after treatment with KEYTRUDA. Consider the benefit of treatment with KEYTRUDA versus the risk of possible GVHD in patients with a history of allogeneic HSCT.
5.10 Increased Mortality in Patients with Multiple Myeloma when KEYTRUDA is Added to a Thalidomide Analogue and Dexamethasone
In two randomized trials in patients with multiple myeloma, the addition of KEYTRUDA to a thalidomide analogue plus dexamethasone, a use for which no PD-1 or PD-L1 blocking antibody is indicated, resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled trials.
5.11 Embryo-Fetal Toxicity
Based on its mechanism of action, KEYTRUDA can cause fetal harm when administered to a pregnant woman. Animal models link the PD-1/PD-L1 signaling pathway with maintenance of pregnancy through induction of maternal immune tolerance to fetal tissue. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with KEYTRUDA and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Immune-mediated pneumonitis [see Warnings and Precautions (5.1)].
- Immune-mediated colitis [see Warnings and Precautions (5.2)].
- Immune-mediated hepatitis (KEYTRUDA) and hepatotoxicity (KEYTRUDA in combination with axitinib) [see Warnings and Precautions (5.3)].
- Immune-mediated endocrinopathies [see Warnings and Precautions (5.4)].
- Immune-mediated nephritis and renal dysfunction [see Warnings and Precautions (5.5)].
- Immune-mediated skin adverse reactions [see Warnings and Precautions (5.6)].
- Other immune-mediated adverse reactions [see Warnings and Precautions (5.7)].
- Infusion-related reactions [see Warnings and Precautions (5.8)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to KEYTRUDA as a single agent in 2799 patients in three randomized, open-label, active-controlled trials (KEYNOTE-002, KEYNOTE-006, and KEYNOTE-010), which enrolled 912 patients with melanoma and 682 patients with NSCLC, and one single-arm trial (KEYNOTE-001), which enrolled 655 patients with melanoma and 550 patients with NSCLC. In addition to the 2799 patients, certain subsections in the WARNINGS AND PRECAUTIONS describe adverse reactions observed with exposure to KEYTRUDA as a single agent in two randomized, open-label, active-controlled clinical trials (KEYNOTE-042 and KEYNOTE-024), which enrolled 790 patients with NSCLC; in a non-randomized, open-label, multi-cohort trial (KEYNOTE-012), a non-randomized, open-label, single-cohort trial (KEYNOTE-055), and two randomized, open-label, active-controlled trials (KEYNOTE-040 and KEYNOTE-048 single agent arms), which enrolled 909 patients with HNSCC; in two non-randomized, open-label trials (KEYNOTE-013 and KEYNOTE-087), which enrolled 241 patients with cHL; in combination with chemotherapy in a randomized, active-controlled trial (KEYNOTE-189), which enrolled 405 patients with nonsquamous NSCLC; in a randomized, open-label, active-controlled trial (KEYNOTE-048 combination arm), which enrolled 276 patients with HNSCC; in combination with axitinib in a randomized, active-controlled trial (KEYNOTE 426), which enrolled 429 patients with RCC; and in post-marketing use. Across all trials, KEYTRUDA was administered at doses of 2 mg/kg intravenously every 3 weeks, 10 mg/kg intravenously every 2 weeks, 10 mg/kg intravenously every 3 weeks, or 200 mg intravenously every 3 weeks. Among the 2799 patients, 41% were exposed for 6 months or more and 21% were exposed for 12 months or more.
The data described in this section were obtained in ten randomized, controlled trials (KEYNOTE-002, KEYNOTE-006, KEYNOTE-010, KEYNOTE-042, KEYNOTE-045, KEYNOTE-048, KEYNOTE-189, KEYNOTE-407, KEYNOTE-181, and KEYNOTE-426) and eleven non-randomized, open-label trials (KEYNOTE-028, KEYNOTE-012, KEYNOTE-087, KEYNOTE-170, KEYNOTE-052, KEYNOTE-057, KEYNOTE-059, KEYNOTE-158, KEYNOTE-224, KEYNOTE-017, and KEYNOTE-146). The data described in this section also included a single randomized, double-blind, placebo-controlled trial (KEYNOTE-054) in which KEYTRUDA was administered for the adjuvant treatment of 509 patients with melanoma with involvement of lymph node(s) following complete surgical resection. In these trials, KEYTRUDA was administered at 2 mg/kg every 3 weeks, 200 mg every 3 weeks, or 10 mg/kg every 2 or 3 weeks.
Melanoma
Ipilimumab-Naive Melanoma
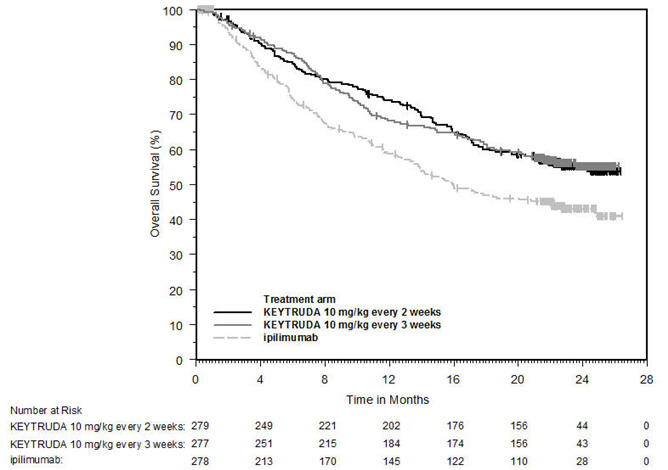
The safety of KEYTRUDA for the treatment of patients with unresectable or metastatic melanoma who had not received prior ipilimumab and who had received no more than one prior systemic therapy was investigated in KEYNOTE-006. KEYNOTE-006 was a multicenter, open-label, active-controlled trial where patients were randomized (1:1:1) and received KEYTRUDA 10 mg/kg every 2 weeks (n=278) or KEYTRUDA 10 mg/kg every 3 weeks (n=277) until disease progression or unacceptable toxicity or ipilimumab 3 mg/kg every 3 weeks for 4 doses unless discontinued earlier for disease progression or unacceptable toxicity (n=256) [see Clinical Studies (14.1)]. Patients with autoimmune disease, a medical condition that required systemic corticosteroids or other immunosuppressive medication; a history of interstitial lung disease; or active infection requiring therapy, including HIV or hepatitis B or C, were ineligible.
The median duration of exposure was 5.6 months (range: 1 day to 11.0 months) for KEYTRUDA and similar in both treatment arms. Fifty-one and 46% of patients received KEYTRUDA 10 mg/kg every 2 or 3 weeks, respectively, for ≥6 months. No patients in either arm received treatment for more than one year.
The study population characteristics were: median age of 62 years (range: 18 to 89); 60% male; 98% White; 32% had an elevated lactate dehydrogenase (LDH) value at baseline; 65% had M1c stage disease; 9% with history of brain metastasis; and approximately 36% had been previously treated with systemic therapy which included a BRAF inhibitor (15%), chemotherapy (13%), and immunotherapy (6%).
In KEYNOTE-006, the adverse reaction profile was similar for the every 2 week and every 3 week schedule, therefore summary safety results are provided in a pooled analysis (n=555) of both KEYTRUDA arms. Adverse reactions leading to permanent discontinuation of KEYTRUDA occurred in 9% of patients. Adverse reactions leading to discontinuation of KEYTRUDA in more than one patient were colitis (1.4%), autoimmune hepatitis (0.7%), allergic reaction (0.4%), polyneuropathy (0.4%), and cardiac failure (0.4%). Adverse reactions leading to interruption of KEYTRUDA occurred in 21% of patients; the most common (≥1%) was diarrhea (2.5%). Tables 2 and 3 summarize selected adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-006.
Table 2: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-006 Adverse Reaction KEYTRUDA
10 mg/kg every 2 or 3 weeksIpilimumab n=555 n=256 All Grades†
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in the ipilimumab arm
- † Graded per NCI CTCAE v4.0
- ‡ Includes rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and exfoliative rash.
- § Includes skin hypopigmentation
General Fatigue 28 0.9 28 3.1 Skin and Subcutaneous Tissue Rash‡ 24 0.2 23 1.2 Vitiligo§ 13 0 2 0 Musculoskeletal and Connective Tissue Arthralgia 18 0.4 10 1.2 Back pain 12 0.9 7 0.8 Respiratory, Thoracic and Mediastinal Cough 17 0 7 0.4 Dyspnea 11 0.9 7 0.8 Metabolism and Nutrition Decreased appetite 16 0.5 14 0.8 Nervous System Headache 14 0.2 14 0.8 Other clinically important adverse reactions occurring in ≥10% of patients receiving KEYTRUDA were diarrhea (26%), nausea (21%), and pruritus (17%).
Table 3: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving KEYTRUDA in KEYNOTE-006 Laboratory Test† KEYTRUDA
10 mg/kg every 2 or 3 weeksIpilimumab All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in ipilimumab arm
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (520 to 546 patients) and ipilimumab (237 to 247 patients); hypertriglyceridemia: KEYTRUDA n=429 and ipilimumab n=183; hypercholesterolemia: KEYTRUDA n=484 and ipilimumab n=205.
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 45 4.2 45 3.8 Hypertriglyceridemia 43 2.6 31 1.1 Hyponatremia 28 4.6 26 7 Increased AST 27 2.6 25 2.5 Hypercholesterolemia 20 1.2 13 0 Hematology Anemia 35 3.8 33 4.0 Lymphopenia 33 7 25 6 Other laboratory abnormalities occurring in ≥20% of patients receiving KEYTRUDA were increased hypoalbuminemia (27% all Grades; 2.4% Grades 3-4), increased ALT (23% all Grades; 3.1% Grades 3-4), and increased alkaline phosphatase (21% all Grades, 2% Grades 3-4).
Ipilimumab-Refractory Melanoma
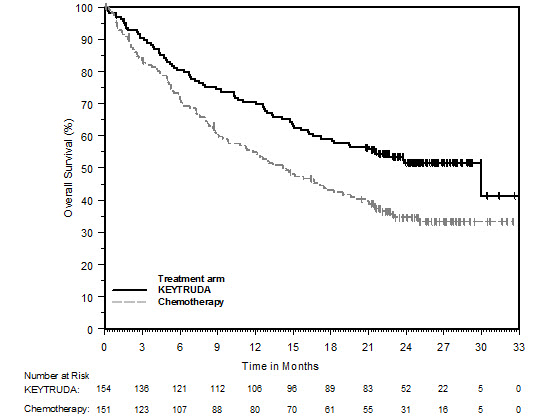
The safety of KEYTRUDA in patients with unresectable or metastatic melanoma with disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor, was investigated in KEYNOTE-002. KEYNOTE-002 was a multicenter, partially blinded (KEYTRUDA dose), randomized (1:1:1), active-controlled trial in which 528 patients received KEYTRUDA 2 mg/kg (n=178) or 10 mg/kg (n=179) every 3 weeks or investigator's choice of chemotherapy (n=171), consisting of dacarbazine (26%), temozolomide (25%), paclitaxel and carboplatin (25%), paclitaxel (16%), or carboplatin (8%) [see Clinical Studies (14.1)]. Patients with autoimmune disease, severe immune-related toxicity related to ipilimumab, defined as any Grade 4 toxicity or Grade 3 toxicity requiring corticosteroid treatment (greater than 10 mg/day prednisone or equivalent dose) for greater than 12 weeks; medical conditions that required systemic corticosteroids or other immunosuppressive medication; a history of interstitial lung disease; or an active infection requiring therapy, including HIV or hepatitis B or C, were ineligible.
The median duration of exposure to KEYTRUDA 2 mg/kg every 3 weeks was 3.7 months (range: 1 day to 16.6 months) and to KEYTRUDA 10 mg/kg every 3 weeks was 4.8 months (range: 1 day to 16.8 months). In the KEYTRUDA 2 mg/kg arm, 36% of patients were exposed to KEYTRUDA for ≥6 months and 4% were exposed for ≥12 months. In the KEYTRUDA 10 mg/kg arm, 41% of patients were exposed to KEYTRUDA for ≥6 months and 6% of patients were exposed to KEYTRUDA for ≥12 months.
The study population characteristics were: median age of 62 years (range: 15 to 89); 61% male; 98% White; 41% had an elevated LDH value at baseline; 83% had M1c stage disease; 73% received two or more prior therapies for advanced or metastatic disease (100% received ipilimumab and 25% a BRAF inhibitor); and 15% with history of brain metastasis.
In KEYNOTE-002, the adverse reaction profile was similar for the 2 mg/kg dose and 10 mg/kg dose, therefore summary safety results are provided in a pooled analysis (n=357) of both KEYTRUDA arms. Adverse reactions resulting in permanent discontinuation occurred in 12% of patients receiving KEYTRUDA; the most common (≥1%) were general physical health deterioration (1%), asthenia (1%), dyspnea (1%), pneumonitis (1%), and generalized edema (1%). Adverse reactions leading to interruption of KEYTRUDA occurred in 14% of patients; the most common (≥1%) were dyspnea (1%), diarrhea (1%), and maculo-papular rash (1%). Tables 4 and 5 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-002.
Table 4: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-002 Adverse Reaction KEYTRUDA
2 mg/kg or 10 mg/kg every 3 weeksChemotherapy† n=357 n=171 All Grades‡
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in chemotherapy arm
- † Chemotherapy: dacarbazine, temozolomide, carboplatin plus paclitaxel, paclitaxel, or carboplatin
- ‡ Graded per NCI CTCAE v4.0
- § Includes rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, and rash pruritic
Skin and Subcutaneous Tissue Pruritus 28 0 8 0 Rash§ 24 0.6 8 0 Gastrointestinal Constipation 22 0.3 20 2.3 Diarrhea 20 0.8 20 2.3 Abdominal pain 13 1.7 8 1.2 Respiratory, Thoracic and Mediastinal Cough 18 0 16 0 General Pyrexia 14 0.3 9 0.6 Asthenia 10 2.0 9 1.8 Musculoskeletal and Connective Tissue Arthralgia 14 0.6 10 1.2 Other clinically important adverse reactions occurring in patients receiving KEYTRUDA were fatigue (43%), nausea (22%), decreased appetite (20%), vomiting (13%), and peripheral neuropathy (1.7%).
Table 5: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving KEYTRUDA in KEYNOTE-002 Laboratory Test† KEYTRUDA
2 mg/kg or 10 mg/kg every 3 weeksChemotherapy All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in chemotherapy arm.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 320 to 325 patients) and chemotherapy (range: 154 to 161 patients); hypertriglyceridemia: KEYTRUDA n=247 and chemotherapy n=116; decreased bicarbonate: KEYTRUDA n=263 and chemotherapy n=123.
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 49 6 44 6 Hypoalbuminemia 37 1.9 33 0.6 Hyponatremia 37 7 24 3.8 Hypertriglyceridemia 33 0 32 0.9 Increased alkaline phosphatase 26 3.1 18 1.9 Increased AST 24 2.2 16 0.6 Decreased bicarbonate 22 0.4 13 0 Hypocalcemia 21 0.3 18 1.9 Increased ALT 21 1.8 16 0.6 Other laboratory abnormalities occurring in ≥20% of patients receiving KEYTRUDA were anemia (44% all Grades; 10% Grades 3-4) and lymphopenia (40% all Grades; 9% Grades 3-4).
Adjuvant Treatment of Resected Melanoma
The safety of KEYTRUDA as a single agent was investigated in KEYNOTE-054, a randomized (1:1) double-blind trial in which 1019 patients with completely resected stage IIIA (>1 mm lymph node metastasis), IIIB or IIIC melanoma received 200 mg of KEYTRUDA by intravenous infusion every 3 weeks (n=509) or placebo (n=502) for up to one year [see Clinical Studies (14.1)]. Patients with active autoimmune disease or a medical condition that required immunosuppression or mucosal or ocular melanoma were ineligible. Seventy-six percent of patients received KEYTRUDA for 6 months or longer.
The study population characteristics were: median age of 54 years (range: 19 to 88), 25% age 65 or older; 62% male; and 94% ECOG PS of 0 and 6% ECOG PS of 1. Sixteen percent had stage IIIA, 46% had stage IIIB, 18% had stage IIIC (1-3 positive lymph nodes), and 20% had stage IIIC (≥4 positive lymph nodes).
Two patients treated with KEYTRUDA died from causes other than disease progression; causes of death were drug reaction with eosinophilia and systemic symptoms and autoimmune myositis with respiratory failure. Serious adverse reactions occurred in 25% of patients receiving KEYTRUDA. Adverse reactions leading to permanent discontinuation occurred in 14% of patients receiving KEYTRUDA; the most common (≥1%) were pneumonitis (1.4%), colitis (1.2%), and diarrhea (1%). Adverse reactions leading to interruption of KEYTRUDA occurred in 19% of patients; the most common (≥1%) were diarrhea (2.4%), pneumonitis (2%), increased ALT (1.4%), arthralgia (1.4%), increased AST (1.4%), dyspnea (1%), and fatigue (1%). Tables 6 and 7 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-054.
Table 6: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-054 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
n=509Placebo
n=502All Grades†
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in placebo arm
- † Graded per NCI CTCAE v4.03
Gastrointestinal Diarrhea 28 1.2 26 1.2 Nausea 17 0.2 15 0 Skin and Subcutaneous Tissue Pruritus 19 0 12 0 Rash 13 0.2 9 0 Musculoskeletal and Connective Tissue Arthralgia 16 1.2 14 0 Endocrine Hypothyroidism 15 0 2.8 0 Hyperthyroidism 10 0.2 1.2 0 Respiratory, Thoracic and Mediastinal Cough 14 0 11 0 General Asthenia 11 0.2 8 0 Influenza like illness 11 0 8 0 Investigations Weight loss 11 0 8 0 Table 7: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving KEYTRUDA in KEYNOTE-054 Laboratory Test† KEYTRUDA
200 mg every 3 weeksPlacebo All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than placebo.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 503 to 507 patients) and placebo (range: 492 to 498 patients).
- ‡ Graded per NCI CTCAE v4.03
Chemistry Increased ALT 27 2.4 16 0.2 Increased AST 24 1.8 15 0.4 Hematology Lymphopenia 24 1 16 1.2 NSCLC
First-line treatment of metastatic nonsquamous NSCLC with pemetrexed and platinum chemotherapy
The safety of KEYTRUDA in combination with pemetrexed and investigator's choice of platinum (either carboplatin or cisplatin) was investigated in KEYNOTE-189, a multicenter, double-blind, randomized (2:1), active-controlled trial in patients with previously untreated, metastatic nonsquamous NSCLC with no EGFR or ALK genomic tumor aberrations [see Clinical Studies (14.2)]. A total of 607 patients received KEYTRUDA 200 mg, pemetrexed and platinum every 3 weeks for 4 cycles followed by KEYTRUDA and pemetrexed (n=405) or placebo, pemetrexed, and platinum every 3 weeks for 4 cycles followed by placebo and pemetrexed (n=202). Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to KEYTRUDA 200 mg every 3 weeks was 7.2 months (range: 1 day to 20.1 months). Sixty percent of patients in the KEYTRUDA arm were exposed to KEYTRUDA for ≥6 months. Seventy-two percent of patients received carboplatin.
The study population characteristics were: median age of 64 years (range: 34 to 84), 49% age 65 or older; 59% male; 94% White and 3% Asian; and 18% with history of brain metastases at baseline.
KEYTRUDA was discontinued for adverse reactions in 20% of patients. The most common adverse reactions resulting in permanent discontinuation of KEYTRUDA were pneumonitis (3%) and acute kidney injury (2%). Adverse reactions leading to the interruption of KEYTRUDA occurred in 53% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of KEYTRUDA (≥2%) were neutropenia (13%), asthenia/fatigue (7%), anemia (7%), thrombocytopenia (5%), diarrhea (4%), pneumonia (4%), increased blood creatinine (3%), dyspnea (2%), febrile neutropenia (2%), upper respiratory tract infection (2%), increased ALT (2%), and pyrexia (2%). Tables 8 and 9 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-189.
Table 8: Adverse Reactions Occurring in ≥20% of Patients in KEYNOTE-189 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
Pemetrexed
Platinum Chemotherapy
n=405Placebo
Pemetrexed
Platinum Chemotherapy
n=202All Grades*
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes asthenia and fatigue
- ‡ Includes genital rash, rash, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular.
Gastrointestinal Nausea 56 3.5 52 3.5 Constipation 35 1.0 32 0.5 Diarrhea 31 5 21 3.0 Vomiting 24 3.7 23 3.0 General Fatigue† 56 12 58 6 Pyrexia 20 0.2 15 0 Metabolism and Nutrition Decreased appetite 28 1.5 30 0.5 Skin and Subcutaneous Tissue Rash‡ 25 2.0 17 2.5 Respiratory, Thoracic and Mediastinal Cough 21 0 28 0 Dyspnea 21 3.7 26 5 Table 9: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients in KEYNOTE-189 Laboratory Test* KEYTRUDA
200 mg every 3 weeks
Pemetrexed
Platinum ChemotherapyPlacebo
Pemetrexed
Platinum ChemotherapyAll Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA/pemetrexed/platinum chemotherapy (range: 381 to 401 patients) and placebo/pemetrexed/platinum chemotherapy (range: 184 to 197 patients).
- † Graded per NCI CTCAE v4.03
Hematology Anemia 85 17 81 18 Lymphopenia 64 22 64 25 Neutropenia 48 20 41 19 Thrombocytopenia 30 12 29 8 Chemistry Hyperglycemia 63 9 60 7 Increased ALT 47 3.8 42 2.6 Increased AST 47 2.8 40 1.0 Hypoalbuminemia 39 2.8 39 1.1 Increased creatinine 37 4.2 25 1.0 Hyponatremia 32 7 23 6 Hypophosphatemia 30 10 28 14 Increased alkaline phosphatase 26 1.8 29 2.1 Hypocalcemia 24 2.8 17 0.5 Hyperkalemia 24 2.8 19 3.1 Hypokalemia 21 5 20 5 First-line treatment of metastatic squamous NSCLC with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy
The safety of KEYTRUDA in combination with carboplatin and investigator's choice of either paclitaxel or paclitaxel protein-bound was investigated in KEYNOTE-407, a multicenter, double-blind, randomized (1:1), placebo-controlled trial in 558 patients with previously untreated, metastatic squamous NSCLC [see Clinical Studies (14.2)]. Safety data are available for the first 203 patients who received KEYTRUDA and chemotherapy (n=101) or placebo and chemotherapy (n=102). Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to KEYTRUDA was 7 months (range: 1 day to 12 months). Sixty-one percent of patients in the KEYTRUDA arm were exposed to KEYTRUDA for ≥6 months. A total of 139 of 203 patients (68%) received paclitaxel and 64 patients (32%) received paclitaxel protein-bound in combination with carboplatin.
The study population characteristics were: median age of 65 years (range: 40 to 83), 52% age 65 or older; 78% male; 83% White; and 9% with history of brain metastases.
KEYTRUDA was discontinued for adverse reactions in 15% of patients, with no single type of adverse reaction accounting for the majority. Adverse reactions leading to interruption of KEYTRUDA occurred in 43% of patients; the most common (≥2%) were thrombocytopenia (20%), neutropenia (11%), anemia (6%), asthenia (2%), and diarrhea (2%). The most frequent (≥2%) serious adverse reactions were febrile neutropenia (6%), pneumonia (6%), and urinary tract infection (3%).
The adverse reactions observed in KEYNOTE-407 were similar to those observed in KEYNOTE-189 with the exception that increased incidences of alopecia (47% vs. 36%) and peripheral neuropathy (31% vs. 25%) were observed in the KEYTRUDA and chemotherapy arm compared to the placebo and chemotherapy arm in KEYNOTE-407.
Previously Untreated NSCLC
The safety of KEYTRUDA was investigated in KEYNOTE-042, a multicenter, open-label, randomized (1:1), active-controlled trial in 1251 patients with PD-L1 expressing, previously untreated stage III NSCLC who were not candidates for surgical resection or definitive chemoradiation or metastatic NSCLC [see Clinical Studies (14.2)]. Patients received KEYTRUDA 200 mg every 3 weeks (n=636) or investigator's choice of chemotherapy (n=615), consisting of pemetrexed and carboplatin followed by optional pemetrexed (n=312) or paclitaxel and carboplatin followed by optional pemetrexed (n=303) every 3 weeks. Patients with EGFR or ALK genomic tumor aberrations; autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to KEYTRUDA was 5.6 months (range: 1 day to 27.3 months). Forty-eight percent of patients in the KEYTRUDA arm were exposed to KEYTRUDA 200 mg for ≥6 months.
The study population characteristics were: median age of 63 years (range: 25 to 90), 45% age 65 or older; 71% male; and 64% White, 30% Asian, and 2% Black. Nineteen percent were Hispanic or Latino. Eighty-seven percent had metastatic disease (stage IV), 13% had stage III disease (2% stage IIIA and 11% stage IIIB), and 5% had treated brain metastases at baseline.
KEYTRUDA was discontinued for adverse reactions in 19% of patients. The most common adverse reactions resulting in permanent discontinuation of KEYTRUDA were pneumonitis (3.0%), death due to unknown cause (1.6%), and pneumonia (1.4%). Adverse reactions leading to interruption of KEYTRUDA occurred in 33% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of KEYTRUDA (≥2%) were pneumonitis (3.1%), pneumonia (3.0%), hypothyroidism (2.2%), and increased ALT (2.0%). The most frequent (≥2%) serious adverse reactions were pneumonia (7%), pneumonitis (3.9%), pulmonary embolism (2.4%), and pleural effusion (2.2%).
Tables 10 and 11 summarize the adverse reactions and laboratory abnormalities, respectively, in patients treated with KEYTRUDA in KEYNOTE-042.
Table 10: Adverse Reactions Occurring in ≥10% of Patients in KEYNOTE-042 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
n=636Chemotherapy
n=615All Grades*
(%)Grades 3-5
(%)All Grades
(%)Grades 3-5
(%)- * Graded per NCI CTCAE v4.03
- † Includes fatigue and asthenia
- ‡ Includes rash, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular.
General Fatigue† 25 3.1 33 3.9 Pyrexia 10 0.3 8 0 Metabolism and Nutrition Decreased appetite 17 1.7 21 1.5 Respiratory, Thoracic and Mediastinal Dyspnea 17 2.0 11 0.8 Cough 16 0.2 11 0.3 Skin and Subcutaneous Tissue Rash‡ 15 1.3 8 0.2 Gastrointestinal Constipation 12 0 21 0.2 Diarrhea 12 0.8 12 0.5 Nausea 12 0.5 32 1.1 Endocrine Hypothyroidism 12 0.2 1.5 0 Infections Pneumonia 12 7 9 6 Investigations Weight loss 10 0.9 7 0.2 Table 11: Laboratory Abnormalities Worsened from Baseline in ≥20% of Patients in KEYNOTE-042 Laboratory Test* KEYTRUDA
200 mg every 3 weeksChemotherapy All Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 598 to 610 patients) and chemotherapy (range: 588 to 597 patients); increased prothrombin INR: KEYTRUDA n=203 and chemotherapy n=173.
- † Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 52 4.7 51 5 Increased ALT 33 4.8 34 2.9 Hypoalbuminemia 33 2.2 29 1.0 Increased AST 31 3.6 32 1.7 Hyponatremia 31 9 32 8 Increased alkaline phosphatase 29 2.3 29 0.3 Hypocalcemia 25 2.5 19 0.7 Hyperkalemia 23 3.0 20 2.2 Increased prothrombin INR 21 2.0 15 2.9 Hematology Anemia 43 4.4 79 19 Lymphopenia 30 7 41 13 Previously Treated NSCLC
The safety of KEYTRUDA was investigated in KEYNOTE-010, a multicenter, open-label, randomized (1:1:1), active-controlled trial, in patients with advanced NSCLC who had documented disease progression following treatment with platinum-based chemotherapy and, if positive for EGFR or ALK genetic aberrations, appropriate therapy for these aberrations [see Clinical Studies (14.2)]. A total of 991 patients received KEYTRUDA 2 mg/kg (n=339) or 10 mg/kg (n=343) every 3 weeks or docetaxel (n=309) at 75 mg/m2 every 3 weeks. Patients with autoimmune disease, medical conditions that required systemic corticosteroids or other immunosuppressive medication, or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to KEYTRUDA 2 mg/kg every 3 weeks was 3.5 months (range: 1 day to 22.4 months) and to KEYTRUDA 10 mg/kg every 3 weeks was 3.5 months (range 1 day to 20.8 months). The data described below reflect exposure to KEYTRUDA 2 mg/kg in 31% of patients exposed to KEYTRUDA for ≥6 months. In the KEYTRUDA 10 mg/kg arm, 34% of patients were exposed to KEYTRUDA for ≥6 months.
The study population characteristics were: median age of 63 years (range: 20 to 88), 42% age 65 or older; 61% male; 72% White and 21% Asian; and 8% with advanced localized disease, 91% with metastatic disease, and 15% with history of brain metastases. Twenty-nine percent received two or more prior systemic treatments for advanced or metastatic disease.
In KEYNOTE-010, the adverse reaction profile was similar for the 2 mg/kg and 10 mg/kg dose, therefore summary safety results are provided in a pooled analysis (n=682). Treatment was discontinued for adverse reactions in 8% of patients receiving KEYTRUDA. The most common adverse events resulting in permanent discontinuation of KEYTRUDA was pneumonitis (1.8%). Adverse reactions leading to interruption of KEYTRUDA occurred in 23% of patients; the most common (≥1%) were diarrhea (1%), fatigue (1.3%), pneumonia (1%), liver enzyme elevation (1.2%), decreased appetite (1.3%), and pneumonitis (1%). Tables 12 and 13 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-010.
Table 12: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-010 Adverse Reaction KEYTRUDA
2 or 10 mg/kg every 3 weeks
n=682Docetaxel
75 mg/m2 every 3 weeks
n=309All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in docetaxel arm
- † Graded per NCI CTCAE v4.0
- ‡ Includes rash, rash erythematous, rash macular, rash maculo-papular, rash papular, and rash pruritic
Metabolism and Nutrition Decreased appetite 25 1.5 23 2.6 Respiratory, Thoracic and Mediastinal Dyspnea 23 3.7 20 2.6 Cough 19 0.6 14 0 Gastrointestinal Nausea 20 1.3 18 0.6 Constipation 15 0.6 12 0.6 Vomiting 13 0.9 10 0.6 Skin and Subcutaneous Tissue Rash‡ 17 0.4 8 0 Pruritus 11 0 3 0.3 Musculoskeletal and Connective Tissue Arthralgia 11 1.0 9 0.3 Back pain 11 1.5 8 0.3 Other clinically important adverse reactions occurring in patients receiving KEYTRUDA were fatigue (25%), diarrhea (14%), asthenia (11%) and pyrexia (11%).
Table 13: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of NSCLC Patients Receiving KEYTRUDA in KEYNOTE-010 Laboratory Test† KEYTRUDA
2 or 10 mg/kg every 3 weeksDocetaxel
75 mg/m2 every 3 weeksAll Grades‡
%Grades 3-4
%All Grades‡
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in docetaxel arm.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 631 to 638 patients) and docetaxel (range: 274 to 277 patients).
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyponatremia 32 8 27 2.9 Increased alkaline phosphatase 28 3.0 16 0.7 Increased AST 26 1.6 12 0.7 Increased ALT 22 2.7 9 0.4 Other laboratory abnormalities occurring in ≥20% of patients receiving KEYTRUDA were hyperglycemia (44% all Grades; 4.1% Grades 3-4), anemia (37% all Grades; 3.8% Grades 3-4), hypertriglyceridemia (36% all Grades; 1.8% Grades 3-4), lymphopenia (35% all Grades; 9% Grades 3-4), hypoalbuminemia (34% all Grades; 1.6% Grades 3-4), and hypercholesterolemia (20% all Grades; 0.7% Grades 3-4).
SCLC
Among the 131 patients with previously treated SCLC who received KEYTRUDA in KEYNOTE-158 Cohort G (n=107) and KEYNOTE-028 Cohort C1 (n=24) [see Clinical Studies (14.3)], the median duration of exposure to KEYTRUDA was 2 months (range: 1 day to 2.25 years). Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with SCLC were similar to those occurring in patients with other solid tumors who received KEYTRUDA as a single agent.
HNSCC
First-line treatment of metastatic or unresectable, recurrent HNSCC
The safety of KEYTRUDA, as a single agent and in combination with platinum (cisplatin or carboplatin) and FU chemotherapy, was investigated in KEYNOTE-048, a multicenter, open-label, randomized (1:1:1), active-controlled trial in patients with previously untreated, recurrent or metastatic HNSCC [see Clinical Studies (14.4)]. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. A total of 576 patients received KEYTRUDA 200 mg every 3 weeks either as a single agent (n=300) or in combination with platinum and FU (n=276) every 3 weeks for 6 cycles followed by KEYTRUDA, compared to 287 patients who received cetuximab weekly in combination with platinum and FU every 3 weeks for 6 cycles followed by cetuximab.
The median duration of exposure to KEYTRUDA was 3.5 months (range: 1 day to 24.2 months) in the KEYTRUDA single agent arm and was 5.8 months (range: 3 days to 24.2 months) in the combination arm. Seventeen percent of patients in the KEYTRUDA single agent arm and 18% of patients in the combination arm were exposed to KEYTRUDA for ≥12 months. Fifty-seven percent of patients receiving KEYTRUDA in combination with chemotherapy started treatment with carboplatin.
KEYTRUDA was discontinued for adverse reactions in 12% of patients in the KEYTRUDA single agent arm. The most common adverse reactions resulting in permanent discontinuation of KEYTRUDA were sepsis (1.7%) and pneumonia (1.3%). Adverse reactions leading to the interruption of KEYTRUDA occurred in 31% of patients; the most common adverse reactions leading to interruption of KEYTRUDA (≥2%) were pneumonia (2.3%), pneumonitis (2.3%), and hyponatremia (2%).
KEYTRUDA was discontinued for adverse reactions in 16% of patients in the combination arm. The most common adverse reactions resulting in permanent discontinuation of KEYTRUDA were pneumonia (2.5%), pneumonitis (1.8%), and septic shock (1.4%). Adverse reactions leading to the interruption of KEYTRUDA occurred in 45% of patients; the most common adverse reactions leading to interruption of KEYTRUDA (≥2%) were neutropenia (14%), thrombocytopenia (10%), anemia (6%), pneumonia (4.7%), and febrile neutropenia (2.9%).
Tables 14 and 15 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-048.
Table 14: Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-048 KEYTRUDA
200 mg every 3 weeksKEYTRUDA
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FUAdverse Reaction n=300 n=276 n=287 All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.0
- † Includes fatigue, asthenia
- ‡ Includes diarrhea, colitis, hemorrhagic diarrhea, microscopic colitis
- § Includes dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis bullous, dermatitis contact, dermatitis exfoliative, drug eruption, erythema, erythema multiforme, rash, erythematous rash, generalized rash, macular rash, maculo-papular rash, pruritic rash, seborrheic dermatitis
- ¶ Includes cough, productive cough
- # Includes dyspnea, exertional dyspnea
- Þ Includes pneumonia, atypical pneumonia, bacterial pneumonia, staphylococcal pneumonia, aspiration pneumonia, lower respiratory tract infection, lung infection, lung infection pseudomonal
- ß Includes peripheral sensory neuropathy, peripheral neuropathy, hypoesthesia, dysesthesia
- à Includes back pain, musculoskeletal chest pain, musculoskeletal pain, myalgia
General Fatigue† 33 4 49 11 48 8 Pyrexia 13 0.7 16 0.7 12 0 Mucosal inflammation 4.3 1.3 31 10 28 5 Gastrointestinal Constipation 20 0.3 37 0 33 1.4 Nausea 17 0 51 6 51 6 Diarrhea‡ 16 0.7 29 3.3 35 3.1 Vomiting 11 0.3 32 3.6 28 2.8 Dysphagia 8 2.3 12 2.9 10 2.1 Stomatitis 3 0 26 8 28 3.5 Skin Rash§ 20 2.3 17 0.7 70 8 Pruritus 11 0 8 0 10 0.3 Respiratory, Thoracic and Mediastinal Cough¶ 18 0.3 22 0 15 0 Dyspnea# 14 2.0 10 1.8 8 1.0 Endocrine Hypothyroidism 18 0 15 0 6 0 Metabolism and Nutrition Decreased appetite 15 1.0 29 4.7 30 3.5 Weight loss 15 2 16 2.9 21 1.4 Infections PneumoniaÞ 12 7 19 11 13 6 Nervous System Headache 12 0.3 11 0.7 8 0.3 Dizziness 5 0.3 10 0.4 13 0.3 Peripheral sensory neuropathyß 1 0 14 1.1 7 1 Musculoskeletal Myalgiaà 12 1.0 13 0.4 11 0.3 Neck pain 6 0.7 10 1.1 7 0.7 Psychiatric Insomnia 7 0.7 10 0 8 0 Table 15: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving KEYTRUDA in KEYNOTE-048 KEYTRUDA
200 mg every 3 weeksKEYTRUDA
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FULaboratory Test* All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA/chemotherapy (range: 235 to 266 patients), KEYTRUDA (range: 241 to 288 patients), cetuximab/chemotherapy (range: 249 to 282 patients).
- † Graded per NCI CTCAE v4.0
Hematology Lymphopenia 54 25 69 35 74 45 Anemia 52 7 89 28 78 19 Thrombocytopenia 12 3.8 73 18 76 18 Neutropenia 7 1.4 67 35 71 42 Chemistry Hyperglycemia 47 3.8 55 6 66 4.7 Hyponatremia 46 17 56 20 59 20 Hypoalbuminemia 44 3.2 47 4.0 49 1.1 Increased AST 28 3.1 24 2.0 37 3.6 Increased ALT 25 2.1 22 1.6 38 1.8 Increased alkaline phosphatase 25 2.1 27 1.2 33 1.1 Hypercalcemia 22 4.6 16 4.3 13 2.6 Hypocalcemia 22 1.1 32 4 58 7 Hyperkalemia 21 2.8 27 4.3 29 4.3 Hypophosphatemia 20 5 35 12 48 19 Hypokalemia 19 5 34 12 47 15 Increased creatinine 18 1.1 36 2.3 27 2.2 Hypomagnesemia 16 0.4 42 1.7 76 6 Previously treated recurrent or metastatic HNSCC
Among the 192 patients with HNSCC enrolled in KEYNOTE-012 [see Clinical Studies (14.4)], the median duration of exposure to KEYTRUDA was 3.3 months (range: 1 day to 27.9 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible for KEYNOTE-012.
The study population characteristics were: median age of 60 years (range: 20 to 84), 35% age 65 or older; 83% male; and 77% White, 15% Asian, and 5% Black. Sixty-one percent of patients had two or more lines of therapy in the recurrent or metastatic setting, and 95% had prior radiation therapy. Baseline ECOG PS was 0 (30%) or 1 (70%) and 86% had M1 disease.
KEYTRUDA was discontinued due to adverse reactions in 17% of patients. Serious adverse reactions occurred in 45% of patients receiving KEYTRUDA. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia, dyspnea, confusional state, vomiting, pleural effusion, and respiratory failure. The incidence of adverse reactions, including serious adverse reactions, was similar between dosage regimens (10 mg/kg every 2 weeks or 200 mg every 3 weeks); therefore, summary safety results are provided in a pooled analysis. The most common adverse reactions (occurring in ≥20% of patients) were fatigue, decreased appetite, and dyspnea. Adverse reactions occurring in patients with HNSCC were generally similar to those occurring in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent, with the exception of increased incidences of facial edema (10% all Grades; 2.1% Grades 3-4) and new or worsening hypothyroidism [see Warnings and Precautions (5.4)].
cHL
Among the 210 patients with cHL enrolled in KEYNOTE-087 [see Clinical Studies (14.5)], the median duration of exposure to KEYTRUDA was 8.4 months (range: 1 day to 15.2 months). KEYTRUDA was discontinued due to adverse reactions in 5% of patients, and treatment was interrupted due to adverse reactions in 26%. Fifteen percent (15%) of patients had an adverse reaction requiring systemic corticosteroid therapy. Serious adverse reactions occurred in 16% of patients. The most frequent serious adverse reactions (≥1%) included pneumonia, pneumonitis, pyrexia, dyspnea, graft versus host disease and herpes zoster. Two patients died from causes other than disease progression; one from GVHD after subsequent allogeneic HSCT and one from septic shock. Tables 16 and 17 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-087.
Table 16: Adverse Reactions in ≥10% of Patients with cHL in KEYNOTE-087 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
N=210All Grades*
(%)Grade 3
(%)- * Graded per NCI CTCAE v4.0
- † Includes fatigue, asthenia
- ‡ Includes cough, productive cough
- § Includes dyspnea, dyspnea exertional, wheezing
- ¶ Includes back pain, myalgia, bone pain, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, musculoskeletal discomfort, neck pain
- # Includes diarrhea, gastroenteritis, colitis, enterocolitis
- Þ Includes rash, rash maculo-papular, drug eruption, eczema, eczema asteatotic, dermatitis, dermatitis acneiform, dermatitis contact, rash erythematous, rash macular, rash papular, rash pruritic, seborrhoeic dermatitis, dermatitis psoriasiform
- ß Includes neuropathy peripheral, peripheral sensory neuropathy, hypoesthesia, paresthesia, dysesthesia, polyneuropathy
General Fatigue† 26 1.0 Pyrexia 24 1.0 Respiratory, Thoracic and Mediastinal Cough‡ 24 0.5 Dyspnea§ 11 1.0 Musculoskeletal and Connective Tissue Musculoskeletal pain¶ 21 1.0 Arthralgia 10 0.5 Gastrointestinal Diarrhea# 20 1.4 Vomiting 15 0 Nausea 13 0 Skin and Subcutaneous Tissue Rash Þ 20 0.5 Pruritus 11 0 Endocrine Hypothyroidism 14 0.5 Infections Upper respiratory tract infection 13 0 Nervous System Headache 11 0.5 Peripheral neuropathyß 10 0 Other clinically important adverse reactions that occurred in less than 10% of patients on KEYNOTE-087 included infusion reactions (9%), hyperthyroidism (3%), pneumonitis (3%), uveitis and myositis (1% each), and myelitis and myocarditis (0.5% each).
Table 17: Selected Laboratory Abnormalities Worsened from Baseline Occurring in ≥15% of cHL Patients Receiving KEYTRUDA in KEYNOTE-087 Laboratory Test* KEYTRUDA
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 208 to 209 patients)
- † Graded per NCI CTCAE v4.0
- ‡ Includes elevation of AST or ALT
Chemistry Hypertransaminasemia‡ 34 2 Increased alkaline phosphatase 17 0 Increased creatinine 15 0.5 Hematology Anemia 30 6 Thrombocytopenia 27 4 Neutropenia 24 7 Hyperbilirubinemia occurred in less than 15% of patients on KEYNOTE-087 (10% all Grades, 2.4% Grade 3-4).
PMBCL
Among the 53 patients with PMBCL treated in KEYNOTE-170 [see Clinical Studies (14.6)], the median duration of exposure to KEYTRUDA was 3.5 months (range: 1 day to 22.8 months).
KEYTRUDA was discontinued due to adverse reactions in 8% of patients, and treatment was interrupted due to adverse reactions in 15%. Twenty-five percent of patients had an adverse reaction requiring systemic corticosteroid therapy. Serious adverse reactions occurred in 26% of patients, and included arrhythmia (4%), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%). Six (11%) patients died within 30 days of start of treatment. Tables 18 and 19 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-170.
Table 18: Adverse Reactions in ≥10% of Patients with PMBCL in KEYNOTE-170 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
N=53All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.0
- † Includes arthralgia, back pain, myalgia, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, bone pain, neck pain, non-cardiac chest pain
- ‡ Includes nasopharyngitis, pharyngitis, rhinorrhea, rhinitis, sinusitis, upper respiratory tract infection
- § Includes fatigue, asthenia
- ¶ Includes allergic cough, cough, productive cough
- # Includes diarrhea, gastroenteritis
- Þ Includes abdominal pain, abdominal pain upper
- ß Includes atrial fibrillation, sinus tachycardia, supraventricular tachycardia, tachycardia
Musculoskeletal and Connective Tissue Musculoskeletal pain† 30 0 Infections Upper respiratory tract infection‡ 28 0 General Pyrexia 28 0 Fatigue§ 23 2 Respiratory, Thoracic and Mediastinal Cough¶ 26 2 Dyspnea 21 11 Gastrointestinal Diarrhea# 13 2 Abdominal pain Þ 13 0 Nausea 11 0 Cardiac Arrhythmia ß 11 4 Nervous System Headache 11 0 Other clinically important adverse reactions that occurred in less than 10% of patients in KEYNOTE-170 included hypothyroidism (8%), hyperthyroidism and pericarditis (4% each), and thyroiditis, pericardial effusion, pneumonitis, arthritis and acute kidney injury (2% each).
Table 19: Laboratory Abnormalities Worsened from Baseline Occurring in ≥15% of PMBCL Patients Receiving KEYTRUDA in KEYNOTE-170 Laboratory Test* KEYTRUDA
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 44 to 48 patients)
- † Graded per NCI CTCAE v4.0
- ‡ Includes elevation of AST or ALT
Hematology Anemia 47 0 Leukopenia 35 9 Lymphopenia 32 18 Neutropenia 30 11 Chemistry Hyperglycemia 38 4 Hypophosphatemia 29 10 Hypertransaminasemia‡ 27 4 Hypoglycemia 19 0 Increased alkaline phosphatase 17 0 Increased creatinine 17 0 Hypocalcemia 15 4 Hypokalemia 15 4 Urothelial Carcinoma
Cisplatin Ineligible Patients with Urothelial Carcinoma
The safety of KEYTRUDA was investigated in KEYNOTE-052, a single-arm trial that enrolled 370 patients with locally advanced or metastatic urothelial carcinoma who were not eligible for cisplatin-containing chemotherapy. Patients with autoimmune disease or medical conditions that required systemic corticosteroids or other immunosuppressive medications were ineligible [see Clinical Studies (14.7)]. Patients received KEYTRUDA 200 mg every 3 weeks until unacceptable toxicity or either radiographic or clinical disease progression.
The median duration of exposure to KEYTRUDA was 2.8 months (range: 1 day to 15.8 months).
KEYTRUDA was discontinued due to adverse reactions in 11% of patients. Eighteen patients (5%) died from causes other than disease progression. Five patients (1.4%) who were treated with KEYTRUDA experienced sepsis which led to death, and three patients (0.8%) experienced pneumonia which led to death. Adverse reactions leading to interruption of KEYTRUDA occurred in 22% of patients; the most common (≥1%) were liver enzyme increase, diarrhea, urinary tract infection, acute kidney injury, fatigue, joint pain, and pneumonia. Serious adverse reactions occurred in 42% of patients. The most frequent serious adverse reactions (≥2%) were urinary tract infection, hematuria, acute kidney injury, pneumonia, and urosepsis.
Immune-related adverse reactions that required systemic glucocorticoids occurred in 8% of patients, use of hormonal supplementation due to an immune-related adverse reaction occurred in 8% of patients, and 5% of patients required at least one steroid dose ≥40 mg oral prednisone equivalent.
Table 20 summarizes adverse reactions in patients on KEYTRUDA in KEYNOTE-052.
Table 20: Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-052 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
N=370All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.0
- † Includes fatigue, asthenia
- ‡ Includes abdominal pain, pelvic pain, flank pain, abdominal pain lower, tumor pain, bladder pain, hepatic pain, suprapubic pain, abdominal discomfort, abdominal pain upper
- § Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, pain in extremity, spinal pain
- ¶ Includes diarrhea, colitis, enterocolitis, gastroenteritis, frequent bowel movements
- # Includes autoimmune hepatitis, hepatitis, hepatitis toxic, liver injury, increased transaminases, hyperbilirubinemia, increased blood bilirubin, increased alanine aminotransferase, increased aspartate aminotransferase, increased hepatic enzymes, increased liver function tests
- Þ Includes dermatitis, dermatitis bullous, eczema, erythema, rash, rash macular, rash maculo-papular, rash pruritic, rash pustular, skin reaction, dermatitis acneiform, seborrheic dermatitis, palmar-plantar erythrodysesthesia syndrome, rash generalized
- ß Includes oedema peripheral, peripheral swelling
General Fatigue†‡ 38 6 Pyrexia 11 0.5 Weight loss 10 0 Musculoskeletal and Connective Tissue Musculoskeletal pain§ 24 4.9 Arthralgia 10 1.1 Metabolism and Nutrition Decreased appetite 22 1.6 Hyponatremia 10 4.1 Gastrointestinal Constipation 21 1.1 Diarrhea¶ 20 2.4 Nausea 18 1.1 Abdominal pain‡ 18 2.7 Elevated LFTs# 13 3.5 Vomiting 12 0 Skin and Subcutaneous Tissue RashÞ 21 0.5 Pruritus 19 0.3 Edema peripheralß 14 1.1 Infections Urinary tract infection 19 9 Blood and Lymphatic System Anemia 17 7 Respiratory, Thoracic, and Mediastinal Cough 14 0 Dyspnea 11 0.5 Renal and Urinary Increased blood creatinine 11 1.1 Hematuria 13 3.0 Previously Treated Urothelial Carcinoma
The safety of KEYTRUDA for the treatment of patients with locally advanced or metastatic urothelial carcinoma with disease progression following platinum-containing chemotherapy was investigated in KEYNOTE-045. KEYNOTE-045 was a multicenter, open-label, randomized (1:1), active-controlled trial in which 266 patients received KEYTRUDA 200 mg every 3 weeks or investigator's choice of chemotherapy (n=255), consisting of paclitaxel (n=84), docetaxel (n=84) or vinflunine (n=87) [see Clinical Studies (14.7)]. Patients with autoimmune disease or a medical condition that required systemic corticosteroids or other immunosuppressive medications were ineligible.
The median duration of exposure was 3.5 months (range: 1 day to 20 months) in patients who received KEYTRUDA and 1.5 months (range: 1 day to 14 months) in patients who received chemotherapy.
KEYTRUDA was discontinued due to adverse reactions in 8% of patients. The most common adverse reaction resulting in permanent discontinuation of KEYTRUDA was pneumonitis (1.9%). Adverse reactions leading to interruption of KEYTRUDA occurred in 20% of patients; the most common (≥1%) were urinary tract infection (1.5%), diarrhea (1.5%), and colitis (1.1%). Serious adverse reactions occurred in 39% of KEYTRUDA-treated patients. The most frequent serious adverse reactions (≥2%) in KEYTRUDA-treated patients were urinary tract infection, pneumonia, anemia, and pneumonitis. Tables 21 and 22 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-045.
Table 21: Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-045 Adverse Reaction KEYTRUDA
200 mg every 3 weeksChemotherapy* n=266 n=255 All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Chemotherapy: paclitaxel, docetaxel, or vinflunine
- † Graded per NCI CTCAE v4.0
- ‡ Includes asthenia, fatigue, malaise, lethargy
- § Includes back pain, myalgia, bone pain, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, musculoskeletal discomfort, neck pain
- ¶ Includes rash maculo-papular, rash, genital rash, rash erythematous, rash papular, rash pruritic, rash pustular, erythema, drug eruption, eczema, eczema asteatotic, dermatitis contact, dermatitis acneiform, dermatitis, seborrheic keratosis, lichenoid keratosis
- # Includes diarrhea, gastroenteritis, colitis, enterocolitis
- Þ Includes cough, productive cough
- ß Includes dyspnea, dyspnea exertional, wheezing
- à Includes blood urine present, hematuria, chromaturia
General Fatigue‡ 38 4.5 56 11 Pyrexia 14 0.8 13 1.2 Musculoskeletal and Connective Tissue Musculoskeletal pain§ 32 3.0 27 2.0 Skin and Subcutaneous Tissue Pruritus 23 0 6 0.4 Rash¶ 20 0.4 13 0.4 Gastrointestinal Nausea 21 1.1 29 1.6 Constipation 19 1.1 32 3.1 Diarrhea# 18 2.3 19 1.6 Vomiting 15 0.4 13 0.4 Abdominal pain 13 1.1 13 2.7 Infections Urinary tract infection 15 4.9 14 4.3 Metabolism and Nutrition Decreased appetite 21 3.8 21 1.2 Respiratory, Thoracic and Mediastinal CoughÞ 15 0.4 9 0 Dyspneaß 14 1.9 12 1.2 Renal and Urinary Hematuria à 12 2.3 8 1.6 Table 22: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Urothelial Carcinoma Patients Receiving KEYTRUDA in KEYNOTE-045 Laboratory Test* KEYTRUDA
200 mg every 3 weeksChemotherapy All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 240 to 248 patients) and chemotherapy (range: 238 to 244 patients); phosphate decreased: KEYTRUDA n=232 and chemotherapy n=222.
- † Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 52 8 60 7 Anemia 52 13 68 18 Lymphopenia 45 15 53 25 Hypoalbuminemia 43 1.7 50 3.8 Hyponatremia 37 9 47 13 Increased alkaline phosphatase 37 7 33 4.9 Increased creatinine 35 4.4 28 2.9 Hypophosphatemia 29 8 34 14 Increased AST 28 4.1 20 2.5 Hyperkalemia 28 0.8 27 6 Hypocalcemia 26 1.6 34 2.1 BCG-unresponsive High-risk NMIBC
The safety of KEYTRUDA was investigated in KEYNOTE-057, a multicenter, open-label, single-arm trial that enrolled 148 patients with high-risk non-muscle invasive bladder cancer (NMIBC), 96 of whom had BCG-unresponsive carcinoma in situ (CIS) with or without papillary tumors. Patients received KEYTRUDA 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC or progressive disease, or up to 24 months of therapy without disease progression.
The median duration of exposure to KEYTRUDA was 4.3 months (range: 1 day to 25.6 months).
KEYTRUDA was discontinued due to adverse reactions in 11% of patients. The most common adverse (>1%) reaction resulting in permanent discontinuation of KEYTRUDA was pneumonitis (1.4%). Adverse reactions leading to interruption of KEYTRUDA occurred in 22% of patients; the most common (≥2%) were diarrhea (4%) and urinary tract infection (2%). Serious adverse reactions occurred in 28% of KEYTRUDA-treated patients. The most frequent serious adverse reactions (≥2%) in KEYTRUDA-treated patients were pneumonia (3%), cardiac ischemia (2%), colitis (2%), pulmonary embolism (2%), sepsis (2%), and urinary tract infection (2%). Tables 23 and 24 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-057.
Table 23: Adverse Reactions Occurring in ≥10% of Patients Receiving KEYTRUDA in KEYNOTE-057 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
N=148All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.03
- † Includes asthenia, fatigue, malaise
- ‡ Includes oedema peripheral, peripheral swelling
- § Includes diarrhea, gastroenteritis, colitis
- ¶ Includes rash maculo-papular, rash, rash erythematous, rash pruritic, rash pustular, erythema, eczema, eczema asteatotic, lichenoid keratosis, urticaria, dermatitis
- # Includes back pain, myalgia, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, neck pain
- Þ Includes cough, productive cough
General Fatigue† 29 0.7 Peripheral edema‡ 11 0 Gastrointestinal Diarrhea§ 24 2.0 Nausea 13 0 Constipation 12 0 Skin and Subcutaneous Tissue Rash¶ 24 0.7 Pruritus 19 0.7 Musculoskeletal and Connective Tissue Musculoskeletal pain# 19 0 Arthralgia 14 1.4 Renal and Urinary Hematuria 19 1.4 Respiratory, Thoracic, and Mediastinal CoughÞ 19 0 Infections Urinary tract infection 12 2.0 Nasopharyngitis 10 0 Endocrine Hypothyroidism 11 0 Table 24: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of BCG-unresponsive NMIBC Patients Receiving KEYTRUDA in KEYNOTE-057 Laboratory Test* KEYTRUDA
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 124 to 147 patients)
- † Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 59 8 Increased ALT 25 3.4 Hyponatremia 24 7 Hypophosphatemia 24 6 Hypoalbuminemia 24 2.1 Hyperkalemia 23 1.4 Hypocalcemia 22 0.7 Increased AST 20 3.4 Increased creatinine 20 0.7 Hematology Anemia 35 1.4 Lymphopenia 29 1.6 Gastric Cancer
Among the 259 patients with gastric cancer enrolled in KEYNOTE-059 [see Clinical Studies (14.9)], the median duration of exposure to KEYTRUDA was 2.1 months (range: 1 day to 21.4 months). Patients with autoimmune disease or a medical condition that required immunosuppression or with clinical evidence of ascites by physical exam were ineligible. Adverse reactions occurring in patients with gastric cancer were similar to those occurring in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent.
Esophageal Cancer
Among the 314 patients with esophageal cancer enrolled in KEYNOTE-181 [see Clinical Studies (14.10)] treated with KEYTRUDA, the median duration of exposure to KEYTRUDA was 2.1 months (range: 1 day to 24.4 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with esophageal cancer were similar to those occurring in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent.
Cervical Cancer
Among the 98 patients with cervical cancer enrolled in Cohort E of KEYNOTE-158 [see Clinical Studies (14.11)], the median duration of exposure to KEYTRUDA was 2.9 months (range: 1 day to 22.1 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible.
KEYTRUDA was discontinued due to adverse reactions in 8% of patients. Serious adverse reactions occurred in 39% of patients receiving KEYTRUDA. The most frequent serious adverse reactions reported included anemia (7%), fistula (4.1%), hemorrhage (4.1%), and infections [except UTIs] (4.1%). Tables 25 and 26 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in KEYNOTE-158.
Table 25: Adverse Reactions Occurring in ≥10% of Patients with Cervical Cancer in KEYNOTE-158 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
N=98All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.0
- † Includes asthenia, fatigue, lethargy, malaise
- ‡ Includes breast pain, cancer pain, dysesthesia, dysuria, ear pain, gingival pain, groin pain, lymph node pain, oropharyngeal pain, pain, pain of skin, pelvic pain, radicular pain, stoma site pain, toothache
- § Includes edema peripheral, peripheral swelling
- ¶ Includes arthralgia, back pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, myositis, neck pain, non-cardiac chest pain, pain in extremity
- # Includes colitis, diarrhea, gastroenteritis
- Þ Includes abdominal discomfort, abdominal distension, abdominal pain, abdominal pain lower, abdominal pain upper
- ß Includes epistaxis, hematuria, hemoptysis, metrorrhagia, rectal hemorrhage, uterine hemorrhage, vaginal hemorrhage
- à Includes bacterial pyelonephritis, pyelonephritis acute, urinary tract infection, urinary tract infection bacterial, urinary tract infection pseudomonal, urosepsis
- è Includes cellulitis, clostridium difficile infection, device-related infection, empyema, erysipelas, herpes virus infection, infected neoplasm, infection, influenza, lower respiratory tract congestion, lung infection, oral candidiasis, oral fungal infection, osteomyelitis, pseudomonas infection, respiratory tract infection, tooth abscess, upper respiratory tract infection, uterine abscess, vulvovaginal candidiasis
- ð Includes dermatitis, drug eruption, eczema, erythema, palmar-plantar erythrodysesthesia syndrome, rash, rash generalized, rash maculo-papular
General Fatigue† 43 5 Pain‡ 22 2.0 Pyrexia 19 1.0 Edema peripheral§ 15 2.0 Musculoskeletal and Connective Tissue Musculoskeletal pain¶ 27 5 Gastrointestinal Diarrhea# 23 2.0 Abdominal painÞ 22 3.1 Nausea 19 0 Vomiting 19 1.0 Constipation 14 0 Metabolism and Nutrition Decreased appetite 21 0 Vascular Hemorrhageß 19 5 Infections UTIà 18 6 Infection (except UTI)è 16 4.1 Skin and Subcutaneous Tissue Rashð 17 2.0 Endocrine Hypothyroidism 11 0 Nervous System Headache 11 2.0 Respiratory, Thoracic and Mediastinal Dyspnea 10 1.0 Table 26: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients with Cervical Cancer in KEYNOTE-158 Laboratory Test* KEYTRUDA
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA (range: 76 to 79 patients)
- † Graded per NCI CTCAE v4.0
Hematology Anemia 54 24 Lymphopenia 47 9 Chemistry Hypoalbuminemia 44 5 Increased alkaline phosphatase 42 2.6 Hyponatremia 38 13 Hyperglycemia 38 1.3 Increased AST 34 3.9 Increased creatinine 32 5 Hypocalcemia 27 0 Increased ALT 21 3.9 Hypokalemia 20 6 Other laboratory abnormalities occurring in ≥10% of patients receiving KEYTRUDA were hypophosphatemia (19% all Grades; 6% Grades 3-4), increased INR (19% all Grades; 0% Grades 3-4), hypercalcemia (14% all Grades; 2.6% Grades 3-4), platelet count decreased (14% all Grades; 1.3% Grades 3-4), activated partial thromboplastin time prolonged (14% all Grades; 0% Grades 3-4), hypoglycemia (13% all Grades; 1.3% Grades 3-4), white blood cell decreased (13% all Grades; 2.6% Grades 3-4), and hyperkalemia (13% all Grades; 1.3% Grades 3-4).
HCC
Among the 104 patients with HCC who received KEYTRUDA in KEYNOTE-224 [see Clinical Studies (14.12)], the median duration of exposure to KEYTRUDA was 4.2 months (range: 1 day to 1.5 years). Adverse reactions occurring in patients with HCC were generally similar to those in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent, with the exception of increased incidences of ascites (8% Grades 3-4) and immune-mediated hepatitis (2.9%). Laboratory abnormalities (Grades 3-4) that occurred at a higher incidence were elevated AST (20%), ALT (9%), and hyperbilirubinemia (10%).
MCC
Among the 50 patients with MCC enrolled in KEYNOTE-017 [see Clinical Studies (14.13)], the median duration of exposure to KEYTRUDA was 6.6 months (range 1 day to 23.6 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with MCC were similar to those occurring in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent. Laboratory abnormalities (Grades 3-4) that occurred at a higher incidence were elevated AST (11%) and hyperglycemia (19%).
RCC
The safety of KEYTRUDA in combination with axitinib was investigated in KEYNOTE-426 [see Clinical Studies (14.14)]. Patients with medical conditions that required systemic corticosteroids or other immunosuppressive medications or had a history of severe autoimmune disease other than type 1 diabetes, vitiligo, Sjogren's syndrome, and hypothyroidism stable on hormone replacement were ineligible. Patients received KEYTRUDA 200 mg intravenously every 3 weeks and axitinib 5 mg orally twice daily, or sunitinib 50 mg once daily for 4 weeks and then off treatment for 2 weeks. The median duration of exposure to the combination therapy of KEYTRUDA and axitinib was 10.4 months (range: 1 day to 21.2 months).
The study population characteristics were: median age of 62 years (range: 30 to 89), 40% age 65 or older; 71% male; 80% White; and 80% Karnofsky Performance Status (KPS) of 90-100 and 20% KPS of 70-80.
Fatal adverse reactions occurred in 3.3% of patients receiving KEYTRUDA in combination with axitinib. These included 3 cases of cardiac arrest, 2 cases of pulmonary embolism and 1 case each of cardiac failure, death due to unknown cause, myasthenia gravis, myocarditis, Fournier's gangrene, plasma cell myeloma, pleural effusion, pneumonitis, and respiratory failure.
Serious adverse reactions occurred in 40% of patients receiving KEYTRUDA in combination with axitinib. Serious adverse reactions in ≥1% of patients receiving KEYTRUDA in combination with axitinib included hepatotoxicity (7%), diarrhea (4.2%), acute kidney injury (2.3%), dehydration (1%), and pneumonitis (1%).
Permanent discontinuation due to an adverse reaction of either KEYTRUDA or axitinib occurred in 31% of patients; 13% KEYTRUDA only, 13% axitinib only, and 8% both drugs. The most common adverse reaction (>1%) resulting in permanent discontinuation of KEYTRUDA, axitinib, or the combination was hepatotoxicity (13%), diarrhea/colitis (1.9%), acute kidney injury (1.6%), and cerebrovascular accident (1.2%).
Dose interruptions or reductions due to an adverse reaction, excluding temporary interruptions of KEYTRUDA infusions due to infusion-related reactions, occurred in 76% of patients receiving KEYTRUDA in combination with axitinib. This includes interruption of KEYTRUDA in 50% of patients. Axitinib was interrupted in 64% of patients and dose reduced in 22% of patients. The most common adverse reactions (>10%) resulting in interruption of KEYTRUDA were hepatotoxicity (14%) and diarrhea (11%), and the most common adverse reactions (>10%) resulting in either interruption or reduction of axitinib were hepatotoxicity (21%), diarrhea (19%), and hypertension (18%).
The most common adverse reactions (≥20%) in patients receiving KEYTRUDA and axitinib were diarrhea, fatigue/asthenia, hypertension, hypothyroidism, decreased appetite, hepatotoxicity, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation.
Twenty-seven percent (27%) of patients treated with KEYTRUDA in combination with axitinib received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction.
Tables 27 and 28 summarize the adverse reactions and laboratory abnormalities, respectively, that occurred in at least 20% of patients treated with KEYTRUDA and axitinib in KEYNOTE-426.
Table 27: Adverse Reactions Occurring in ≥20% of Patients Receiving KEYTRUDA with Axitinib in KEYNOTE-426 Adverse Reaction KEYTRUDA
200 mg every 3 weeks
and Axitinib
n=429Sunitinib
n=425All Grades*
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes diarrhea, colitis, enterocolitis, gastroenteritis, enteritis, enterocolitis hemorrhagic
- ‡ Includes hypertension, blood pressure increased, hypertensive crisis, labile hypertension
- § Includes ALT increased, AST increased, autoimmune hepatitis, blood bilirubin increased, drug-induced liver injury, hepatic enzyme increased, hepatic function abnormal, hepatitis, hepatitis fulminant, hepatocellular injury, hepatotoxicity, hyperbilirubinemia, immune-mediated hepatitis, liver function test increased, liver injury, transaminases increased
- ¶ Includes rash, butterfly rash, dermatitis, dermatitis acneform, dermatitis atopic, dermatitis bullous, dermatitis contact, exfoliative rash, genital rash, rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, seborrhoeric dermatitis, skin discoloration, skin exfoliation, perineal rash
Gastrointestinal Diarrhea† 56 11 45 5 Nausea 28 0.9 32 0.9 Constipation 21 0 15 0.2 General Fatigue/Asthenia 52 5 51 10 Vascular Hypertension‡ 48 24 48 20 Hepatobiliary Hepatotoxicity§ 39 20 25 4.9 Endocrine Hypothyroidism 35 0.2 32 0.2 Metabolism and Nutrition Decreased appetite 30 2.8 29 0.7 Skin and Subcutaneous Tissue Palmar-plantar erythrodysaesthesia syndrome 28 5 40 3.8 Stomatitis/Mucosal inflammation 27 1.6 41 4 Rash¶ 25 1.4 21 0.7 Respiratory, Thoracic and Mediastinal Dysphonia 25 0.2 3.3 0 Cough 21 0.2 14 0.5 Table 28: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving KEYTRUDA with Axitinib in KEYNOTE-426 Laboratory Test* KEYTRUDA
200 mg every 3 weeks
and AxitinibSunitinib All Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA/axitinib (range: 342 to 425 patients) and sunitinib (range: 345 to 422 patients).
- † Graded per NCI CTCAE v4.03
- ‡ Corrected for albumin
- § Two patients with a Grade 3 elevated activated partial thromboplastin time prolonged (aPTT) were also reported as having an adverse reaction of hepatotoxicity.
Chemistry Hyperglycemia 62 9 54 3.2 Increased ALT 60 20 44 5 Increased AST 57 13 56 5 Increased creatinine 43 4.3 40 2.4 Hyponatremia 35 8 29 8 Hyperkalemia 34 6 22 1.7 Hypoalbuminemia 32 0.5 34 1.7 Hypercalcemia 27 0.7 15 1.9 Hypophosphatemia 26 6 49 17 Increased alkaline phosphatase 26 1.7 30 2.7 Hypocalcemia‡ 22 0.2 29 0.7 Blood bilirubin increased 22 2.1 21 1.9 Activated partial thromboplastin time prolonged§ 22 1.2 14 0 Hematology Lymphopenia 33 11 46 8 Anemia 29 2.1 65 8 Thrombocytopenia 27 1.4 78 14 Endometrial Carcinoma
The safety of KEYTRUDA in combination with lenvatinib (20 mg orally once daily) was investigated in KEYNOTE-146, a single-arm, multicenter, open-label trial in 94 patients with endometrial carcinoma whose tumors had progressed following one line of systemic therapy and were not MSI-H or dMMR [see Clinical Studies (14.15)]. The median duration of study treatment was 7 months (range: 0.03 to 37.8 months). The median duration of exposure to KEYTRUDA was 6 months (range: 0.03 to 23.8 months). KEYTRUDA was continued for a maximum of 24 months; however, treatment with lenvatinib could be continued beyond 24 months.
Fatal adverse reactions occurred in 3% of patients receiving KEYTRUDA and lenvatinib, including gastrointestinal perforation, reversible posterior leukoencephalopathy syndrome (RPLS) with intraventricular hemorrhage, and intracranial hemorrhage.
Serious adverse reactions occurred in 52% of patients receiving KEYTRUDA and lenvatinib. Serious adverse reactions in ≥3% of patients were hypertension (9%), abdominal pain (6%), musculoskeletal pain (5%), hemorrhage (4%), fatigue (4%), nausea (4%), confusional state (4%), pleural effusion (4%), adrenal insufficiency (3%), colitis (3%), dyspnea (3%), and pyrexia (3%).
KEYTRUDA was discontinued for adverse reactions (Grade 1-4) in 19% of patients, regardless of action taken with lenvatinib. The most common adverse reactions (≥ 2%) leading to discontinuation of KEYTRUDA were adrenal insufficiency (2%), colitis (2%), pancreatitis (2%), and muscular weakness (2%).
Adverse reactions leading to interruption of KEYTRUDA occurred in 49% of patients; the most common adverse reactions leading to interruption of KEYTRUDA (≥2%) were: fatigue (14%), diarrhea (6%), decreased appetite (6%), rash (5%), renal impairment (4%), vomiting (4%), increased lipase (4%), decreased weight (4%), nausea (3%), increased blood alkaline phosphatase (3%), skin ulcer (3%), adrenal insufficiency (2%), increased amylase (2%), hypocalcemia (2%), hypomagnesemia (2%), hyponatremia (2%), peripheral edema (2%), musculoskeletal pain (2%), pancreatitis (2%), and syncope (2%).
Tables 29 and 30 summarize adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA in combination with lenvatinib.
Table 29: Adverse Reactions Occurring in ≥20% of Patients with Endometrial Carcinoma in KEYNOTE-146 Adverse Reaction KEYTRUDA
200 mg every 3 weeks with Lenvatinib
N=94All Grades
(%)Grades 3-4
(%)- * Includes asthenia, fatigue, and malaise
- † Includes arthralgia, arthritis, back pain, breast pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, pain in extremity
- ‡ Includes essential hypertension, hypertension, and hypertensive encephalopathy
- § Includes catheter site bruise, contusion, epistaxis, gastrointestinal hemorrhage, hematemesis, hematuria, hemorrhage intracranial, injection site hemorrhage, intraventricular hemorrhage, large intestinal hemorrhage, metrorrhagia, mouth hemorrhage, uterine hemorrhage, and vaginal hemorrhage
- ¶ Includes diarrhea, gastroenteritis, gastrointestinal viral infection, and viral diarrhea
- # Includes glossitis, mouth ulceration, oral discomfort, oral mucosal blistering, oropharyngeal pain, and stomatitis
- Þ Includes abdominal discomfort, abdominal pain, lower abdominal pain, and upper abdominal pain
- ß Includes decreased appetite and early satiety
- à Includes increased blood thyroid stimulating hormone and hypothyroidism
- è Includes cystitis and urinary tract infection
- ð Includes dyspnea and exertional dyspnea
- ø Includes rash, rash generalized, rash macular, and rash maculo-papular
General Fatigue* 65 17 Musculoskeletal and Connective Tissue Musculoskeletal pain† 65 3 Vascular Hypertension‡ 65 38 Hemorrhagic events§ 28 4 Gastrointestinal Diarrhea¶ 64 4 Nausea 48 5 Stomatitis# 43 0 Vomiting 39 0 Abdominal pain Þ 33 6 Constipation 32 0 Metabolism Decreased appetiteß 52 0 Hypomagnesemia 27 3 Endocrine Hypothyroidismà 51 1 Investigations Decreased weight 36 3 Nervous System Headache 33 1 Infections Urinary tract infectionè 31 4 Respiratory, Thoracic and Mediastinal Dysphonia 29 0 Dyspneað 24 2 Cough 21 0 Skin and Subcutaneous Tissue Palmar-plantar erythrodysesthesia syndrome 26 3 Rashø 21 3 Table 30: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% (All Grades) or ≥3% (Grades 3-4) of Patients with Endometrial Carcinoma in KEYNOTE-146 Laboratory Test* KEYTRUDA
200 mg every 3 weeks with LenvatinibAll Grades
%†Grade 3-4
%†- * With at least 1 grade increase from baseline
- † Laboratory abnormality percentage is based on the number of patients who had both baseline and at least one post-baseline laboratory measurement for each parameter (range: 71 to 92 patients).
Chemistry Increased creatinine 80 7 Hypertriglyceridemia 58 4 Hyperglycemia 53 1 Hypercholesteremia 49 6 Hypoalbuminemia 48 0 Hypomagnesemia 47 2 Increased aspartate aminotransferase 43 4 Hyponatremia 42 13 Increased lipase 42 18 Increased alanine aminotransferase 35 3 Increased alkaline phosphatase 32 1 Hypokalemia 27 5 Increased amylase 19 6 Hypocalcemia 14 3 Hypermagnesemia 4 3 Hematology Thrombocytopenia 48 0 Leukopenia 38 2 Lymphopenia 36 7 Anemia 35 1 Increased INR 21 3 Neutropenia 12 3 6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to pembrolizumab in the studies described below with the incidences of antibodies in other studies or to other products may be misleading.
Trough levels of pembrolizumab interfere with the electrochemiluminescent (ECL) assay results; therefore, a subset analysis was performed in the patients with a concentration of pembrolizumab below the drug tolerance level of the anti-product antibody assay. In clinical studies in patients treated with pembrolizumab at a dose of 2 mg/kg every 3 weeks, 200 mg every 3 weeks, or 10 mg/kg every 2 or 3 weeks, 27 (2.1%) of 1289 evaluable patients tested positive for treatment-emergent anti-pembrolizumab antibodies of whom six (0.5%) patients had neutralizing antibodies against pembrolizumab. There was no evidence of an altered pharmacokinetic profile or increased infusion reactions with anti-pembrolizumab binding antibody development.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, KEYTRUDA can cause fetal harm when administered to a pregnant woman. There are no available human data informing the risk of embryo-fetal toxicity. In animal models, the PD-1/PD-L1 signaling pathway is important in the maintenance of pregnancy through induction of maternal immune tolerance to fetal tissue (see Data). Human IgG4 (immunoglobulins) are known to cross the placenta; therefore, pembrolizumab has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with KEYTRUDA to evaluate its effect on reproduction and fetal development. A literature-based assessment of the effects of the PD-1 pathway on reproduction demonstrated that a central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. Blockade of PD-L1 signaling has been shown in murine models of pregnancy to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering KEYTRUDA during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 knockout mice. Based on its mechanism of action, fetal exposure to pembrolizumab may increase the risk of developing immune-mediated disorders or of altering the normal immune response.
8.2 Lactation
Risk Summary
There are no data on the presence of pembrolizumab in either animal or human milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with KEYTRUDA and for 4 months after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating KEYTRUDA [see Use in Specific Populations (8.1)].
Contraception
KEYTRUDA can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions (5.11), Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with KEYTRUDA and for at least 4 months following the final dose.
8.4 Pediatric Use
The safety and effectiveness of KEYTRUDA have been established in pediatric patients with cHL, PMBCL, and MSI-H cancer. Use of KEYTRUDA in pediatric patients with cHL, PMBCL, and MSI-H cancers is supported by evidence from adequate and well-controlled studies of KEYTRUDA in adults with additional pharmacokinetic and safety data in pediatric patients [see Adverse Reactions (6.1), Clinical Studies (14.5, 14.6, 14.8), Clinical Pharmacology (12.3)].
There is limited experience with KEYTRUDA in pediatric patients. In a trial (NCT02332668), 40 pediatric patients (16 children ages 2 years to less than 12 years and 24 adolescents ages 12 years to 18 years) with various cancers, including unapproved usages, were administered KEYTRUDA 2 mg/kg every 3 weeks. Patients received KEYTRUDA for a median of 3 doses (range: 1-17 doses), with 34 patients (85%) receiving KEYTRUDA for 2 doses or more.
The safety profile in these pediatric patients was similar to that seen in adults; adverse reactions that occurred at a higher rate (≥15% difference) in pediatric patients when compared to adults <65 years of age were fatigue (45%), vomiting (38%), abdominal pain (28%), increased transaminases (28%) and hyponatremia (18%).
The concentrations of pembrolizumab in pediatric patients were comparable to those observed in adult patients at the same dose regimen of 2 mg/kg every 3 weeks.
The safety and effectiveness of KEYTRUDA in pediatric patients have not been established in the other approved indications [see Indications and Usage (1)].
8.5 Geriatric Use
Of 3991 patients with melanoma, NSCLC, HNSCC, cHL or urothelial carcinoma who were treated with KEYTRUDA in clinical studies, 46% were 65 years and over and 16% were 75 years and over. No overall differences in safety or effectiveness were observed between elderly patients and younger patients.
-
11 DESCRIPTION
Pembrolizumab is a programmed death receptor-1 (PD 1)-blocking antibody. Pembrolizumab is a humanized monoclonal IgG4 kappa antibody with an approximate molecular weight of 149 kDa. Pembrolizumab is produced in recombinant Chinese hamster ovary (CHO) cells.
KEYTRUDA (pembrolizumab) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for intravenous use. Each vial contains 100 mg of pembrolizumab in 4 mL of solution. Each 1 mL of solution contains 25 mg of pembrolizumab and is formulated in: L-histidine (1.55 mg), polysorbate 80 (0.2 mg), sucrose (70 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
12.2 Pharmacodynamics
Based on dose/exposure efficacy and safety relationships, there are no clinically significant differences in efficacy and safety between pembrolizumab doses of 200 mg or 2 mg/kg every 3 weeks in patients with melanoma or NSCLC.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of pembrolizumab was characterized using a population PK analysis with concentration data collected from 2993 patients with various cancers who received pembrolizumab doses of 1 to 10 mg/kg every 2 weeks, 2 to 10 mg/kg every 3 weeks, or 200 mg every 3 weeks.
Steady-state concentrations of pembrolizumab were reached by 16 weeks of repeated dosing with an every 3-week regimen and the systemic accumulation was 2.1-fold. The peak concentration (Cmax), trough concentration (Cmin), and area under the plasma concentration versus time curve at steady state (AUCss) of pembrolizumab increased dose proportionally in the dose range of 2 to 10 mg/kg every 3 weeks.
Distribution
The geometric mean value (CV%) for volume of distribution at steady state is 6.0 L (20%).
Elimination
Pembrolizumab clearance (CV%) is approximately 23% lower [geometric mean, 195 mL/day (40%)] at steady state than that after the first dose [252 mL/day (37%)]; this decrease in clearance with time is not considered clinically important. The terminal half-life (t1/2) is 22 days (32%).
Specific Populations
The following factors had no clinically important effect on the CL of pembrolizumab: age (range: 15 to 94 years), sex, race (89% White), renal impairment (eGFR ≥ 15 mL/min/1.73 m2), mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and AST > ULN or total bilirubin between 1 and 1.5 times ULN and any AST), or tumor burden. The impact of moderate or severe hepatic impairment on the pharmacokinetics of pembrolizumab is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to test the potential of pembrolizumab for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with pembrolizumab. In 1-month and 6-month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs; however, most animals in these studies were not sexually mature.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-1 signaling resulted in an increased severity of some infections and enhanced inflammatory responses. M. tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 knockout mice have also shown decreased survival following infection with lymphocytic choriomeningitis virus (LCMV). Administration of pembrolizumab in chimpanzees with naturally occurring chronic hepatitis B infection resulted in two out of four animals with significantly increased levels of serum ALT, AST, and GGT, which persisted for at least 1 month after discontinuation of pembrolizumab.
-
14 CLINICAL STUDIES
14.1 Melanoma
Ipilimumab-Naive Melanoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-006 (NCT01866319), a randomized (1:1:1), open-label, multicenter, active-controlled trial in 834 patients. Patients were randomized to receive KEYTRUDA at a dose of 10 mg/kg intravenously every 2 weeks or 10 mg/kg intravenously every 3 weeks until disease progression or unacceptable toxicity or to ipilimumab 3 mg/kg intravenously every 3 weeks for 4 doses unless discontinued earlier for disease progression or unacceptable toxicity. Patients with disease progression could receive additional doses of treatment unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at 4 to 6 weeks with repeat imaging. Randomization was stratified by line of therapy (0 vs. 1), ECOG PS (0 vs. 1), and PD-L1 expression (≥1% of tumor cells [positive] vs. <1% of tumor cells [negative]) according to an investigational use only (IUO) assay. Key eligibility criteria were unresectable or metastatic melanoma; no prior ipilimumab; and no more than one prior systemic treatment for metastatic melanoma. Patients with BRAF V600E mutation-positive melanoma were not required to have received prior BRAF inhibitor therapy. Patients with autoimmune disease; a medical condition that required immunosuppression; previous severe hypersensitivity to other monoclonal antibodies; and HIV, hepatitis B or hepatitis C infection, were ineligible. Assessment of tumor status was performed at 12 weeks, then every 6 weeks through Week 48, followed by every 12 weeks thereafter. The major efficacy outcome measures were overall survival (OS) and progression-free survival (PFS; as assessed by blinded independent central review [BICR] using Response Evaluation Criteria in Solid Tumors [RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ]). Additional efficacy outcome measures were objective response rate (ORR) and duration of response (DoR).
The study population characteristics were: median age of 62 years (range: 18 to 89); 60% male; 98% White; 66% had no prior systemic therapy for metastatic disease; 69% ECOG PS of 0; 80% had PD-L1 positive melanoma, 18% had PD-L1 negative melanoma, and 2% had unknown PD-L1 status using the IUO assay; 65% had M1c stage disease; 68% with normal LDH; 36% with reported BRAF mutation-positive melanoma; and 9% with a history of brain metastases. Among patients with BRAF mutation-positive melanoma, 139 (46%) were previously treated with a BRAF inhibitor.
The study demonstrated statistically significant improvements in OS and PFS for patients randomized to KEYTRUDA as compared to ipilimumab. Among the 91 patients randomized to KEYTRUDA 10 mg/kg every 3 weeks with an objective response, response durations ranged from 1.4+ to 8.1+ months. Among the 94 patients randomized to KEYTRUDA 10 mg/kg every 2 weeks with an objective response, response durations ranged from 1.4+ to 8.2 months. Efficacy results are summarized in Table 31 and Figure 1.
Table 31: Efficacy Results in KEYNOTE-006 Endpoint KEYTRUDA
10 mg/kg every 3 weeks
n=277KEYTRUDA
10 mg/kg every 2 weeks
n=279Ipilimumab
3 mg/kg every 3 weeks
n=278- * Hazard ratio (KEYTRUDA compared to ipilimumab) based on the stratified Cox proportional hazard model
OS Deaths (%) 92 (33%) 85 (30%) 112 (40%) Hazard ratio* (95% CI) 0.69 (0.52, 0.90) 0.63 (0.47, 0.83) --- p-Value (stratified log-rank) 0.004 <0.001 --- PFS by BICR Events (%) 157 (57%) 157 (56%) 188 (68%) Median in months (95% CI) 4.1 (2.9, 6.9) 5.5 (3.4, 6.9) 2.8 (2.8, 2.9) Hazard ratio* (95% CI) 0.58 (0.47, 0.72) 0.58 (0.46, 0.72) --- p-Value (stratified log-rank) <0.001 <0.001 --- Best objective response by BICR ORR (95% CI) 33% (27, 39) 34% (28, 40) 12% (8, 16) Complete response rate 6% 5% 1% Partial response rate 27% 29% 10% Ipilimumab-Refractory Melanoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-002 (NCT01704287), a multicenter, randomized (1:1:1), active-controlled trial in 540 patients randomized to receive one of two doses of KEYTRUDA in a blinded fashion or investigator's choice chemotherapy. The treatment arms consisted of KEYTRUDA 2 mg/kg or 10 mg/kg intravenously every 3 weeks or investigator's choice of any of the following chemotherapy regimens: dacarbazine 1000 mg/m2 intravenously every 3 weeks (26%), temozolomide 200 mg/m2 orally once daily for 5 days every 28 days (25%), carboplatin AUC 6 mg/mL/min intravenously plus paclitaxel 225 mg/m2 intravenously every 3 weeks for four cycles then carboplatin AUC of 5 mg/mL/min plus paclitaxel 175 mg/m2 every 3 weeks (25%), paclitaxel 175 mg/m2 intravenously every 3 weeks (16%), or carboplatin AUC 5 or 6 mg/mL/min intravenously every 3 weeks (8%). Randomization was stratified by ECOG PS (0 vs. 1), LDH levels (normal vs. elevated [≥110% ULN]) and BRAF V600 mutation status (wild-type [WT] or V600E). The trial included patients with unresectable or metastatic melanoma with progression of disease; refractory to two or more doses of ipilimumab (3 mg/kg or higher) and, if BRAF V600 mutation-positive, a BRAF or MEK inhibitor; and disease progression within 24 weeks following the last dose of ipilimumab. The trial excluded patients with uveal melanoma and active brain metastasis. Patients received KEYTRUDA until unacceptable toxicity; disease progression that was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at 4 to 6 weeks with repeat imaging; withdrawal of consent; or physician's decision to stop therapy for the patient. Assessment of tumor status was performed at 12 weeks after randomization, then every 6 weeks through week 48, followed by every 12 weeks thereafter. Patients on chemotherapy who experienced progression of disease were offered KEYTRUDA. The major efficacy outcomes were PFS as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS. Additional efficacy outcome measures were confirmed ORR as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and DoR.
The study population characteristics were: median age of 62 years (range: 15 to 89), 43% age 65 or older; 61% male; 98% White; and 55% ECOG PS of 0 and 45% ECOG PS of 1. Twenty-three percent of patients were BRAF V600 mutation positive, 40% had elevated LDH at baseline, 82% had M1c disease, and 73% had two or more prior therapies for advanced or metastatic disease.
The study demonstrated a statistically significant improvement in PFS for patients randomized to KEYTRUDA as compared to control arm. There was no statistically significant difference between KEYTRUDA 2 mg/kg and chemotherapy or between KEYTRUDA 10 mg/kg and chemotherapy in the OS analysis in which 55% of the patients who had been randomized to receive chemotherapy had crossed over to receive KEYTRUDA. Among the 38 patients randomized to KEYTRUDA 2 mg/kg with an objective response, response durations ranged from 1.3+ to 11.5+ months. Among the 46 patients randomized to KEYTRUDA 10 mg/kg with an objective response, response durations ranged from 1.1+ to 11.1+ months. Efficacy results are summarized in Table 32.
Table 32: Efficacy Results in KEYNOTE-002 Endpoint KEYTRUDA
2 mg/kg every 3 weeksKEYTRUDA
10 mg/kg every 3 weeksChemotherapy n=180 n=181 n=179 - * Hazard ratio (KEYTRUDA compared to chemotherapy) based on the stratified Cox proportional hazard model
- † With additional follow-up of 18 months after the PFS analysis
- ‡ Not statistically significant compared to multiplicity adjusted significance level of 0.01
PFS Number of Events, n (%) 129 (72%) 126 (70%) 155 (87%) Progression, n (%) 105 (58%) 107 (59%) 134 (75%) Death, n (%) 24 (13%) 19 (10%) 21 (12%) Median in months (95% CI) 2.9 (2.8, 3.8) 2.9 (2.8, 4.7) 2.7 (2.5, 2.8) p-Value (stratified log-rank) <0.001 <0.001 --- Hazard ratio* (95% CI) 0.57 (0.45, 0.73) 0.50 (0.39, 0.64) --- OS† Deaths (%) 123 (68%) 117 (65%) 128 (72%) Hazard ratio* (95% CI) 0.86 (0.67, 1.10) 0.74 (0.57, 0.96) --- p-Value (stratified log-rank) 0.117 0.011‡ --- Median in months (95% CI) 13.4 (11.0, 16.4) 14.7 (11.3, 19.5) 11.0 (8.9, 13.8) Objective Response Rate ORR (95% CI) 21% (15, 28) 25% (19, 32) 4% (2, 9) Complete response rate 2% 3% 0% Partial response rate 19% 23% 4% Figure 2: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-002 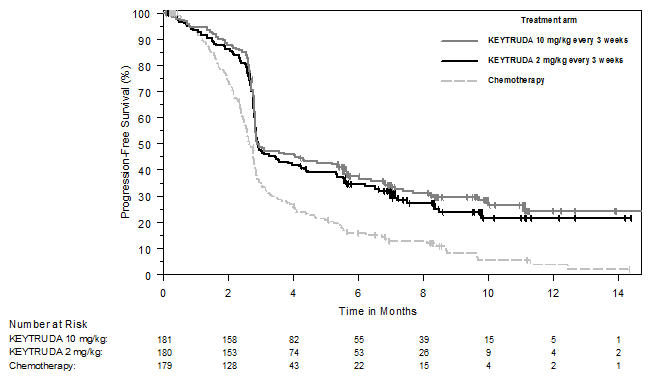
Adjuvant Treatment of Resected Melanoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-054 (NCT02362594), a multicenter, randomized (1:1), double-blind, placebo-controlled trial in patients with completely resected stage IIIA (>1 mm lymph node metastasis), IIIB or IIIC melanoma. Patients were randomized to KEYTRUDA 200 mg intravenously every three weeks or placebo for up to one year until disease recurrence or unacceptable toxicity. Randomization was stratified by American Joint Committee on Cancer 7th edition (AJCC) stage (IIIA vs. IIIB vs. IIIC 1-3 positive lymph nodes vs. IIIC ≥4 positive lymph nodes) and geographic region (North America, European countries, Australia, and other countries as designated). Patients must have undergone lymph node dissection and, if indicated, radiotherapy within 13 weeks prior to starting treatment. The major efficacy outcome measure was investigator-assessed recurrence-free survival (RFS) in the whole population and in the population with PD-L1 positive tumors where RFS was defined as the time between the date of randomization and the date of first recurrence (local, regional, or distant metastasis) or death, whichever occurs first. Patients underwent imaging every 12 weeks after the first dose of KEYTRUDA for the first two years, then every 6 months from year 3 to 5, and then annually.
The study population characteristics were: median age of 54 years (range: 19 to 88), 25% age 65 or older; 62% male; and 94% ECOG PS of 0 and 6% ECOG PS of 1. Sixteen percent had stage IIIA, 46% had stage IIIB, 18% had stage IIIC (1-3 positive lymph nodes), and 20% had stage IIIC (≥4 positive lymph nodes); 50% were BRAF V600 mutation positive and 44% were BRAF wild-type; and 84% had PD-L1 positive melanoma with TPS ≥1% according to an IUO assay.
The trial demonstrated a statistically significant improvement in RFS for patients randomized to the KEYTRUDA arm compared with placebo. Efficacy results are summarized in Table 33 and Figure 3.
Table 33: Efficacy Results in KEYNOTE-054 Endpoint KEYTRUDA
200 mg every 3 weeks
n=514Placebo
n=505NR = not reached - * Based on the stratified Cox proportional hazard model
- † Stratified by American Joint Committee on Cancer 7th edition (AJCC) stage
- ‡ p-Value is compared with 0.008 of the allocated alpha for this interim analysis.
RFS Number (%) of patients with event 135 (26%) 216 (43%) Median in months (95% CI) NR 20.4 (16.2, NR) Hazard ratio*† (95% CI) 0.57 (0.46, 0.70) p-Value† (log-rank) <0.001‡ For patients with PD-L1 positive tumors, the HR was 0.54 (95% CI: 0.42, 0.69); p<0.001. The RFS benefit for KEYTRUDA compared to placebo was observed regardless of tumor PD-L1 expression.
Figure 3: Kaplan-Meier Curve for Recurrence-Free Survival in KEYNOTE-054 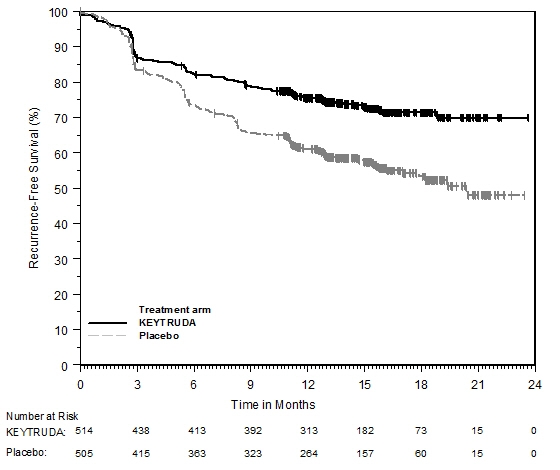
14.2 Non-Small Cell Lung Cancer
First-line treatment of metastatic nonsquamous NSCLC with pemetrexed and platinum chemotherapy
The efficacy of KEYTRUDA in combination with pemetrexed and platinum chemotherapy was investigated in KEYNOTE-189 (NCT02578680), a randomized, multicenter, double-blind, active-controlled trial conducted in 616 patients with metastatic nonsquamous NSCLC, regardless of PD-L1 tumor expression status, who had not previously received systemic therapy for metastatic disease and in whom there were no EGFR or ALK genomic tumor aberrations. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by smoking status (never vs. former/current), choice of platinum (cisplatin vs. carboplatin), and tumor PD-L1 status (TPS <1% [negative] vs. TPS ≥1%). Patients were randomized (2:1) to one of the following treatment arms:
- KEYTRUDA 200 mg, pemetrexed 500 mg/m2, and investigator's choice of cisplatin 75 mg/m2 or carboplatin AUC 5 mg/mL/min intravenously on Day 1 of each 21-day cycle for 4 cycles followed by KEYTRUDA 200 mg and pemetrexed 500 mg/m2 intravenously every 3 weeks. KEYTRUDA was administered prior to chemotherapy on Day 1.
- Placebo, pemetrexed 500 mg/m2, and investigator's choice of cisplatin 75 mg/m2 or carboplatin AUC 5 mg/mL/min intravenously on Day 1 of each 21-day cycle for 4 cycles followed by placebo and pemetrexed 500 mg/m2 intravenously every 3 weeks.
Treatment with KEYTRUDA continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of KEYTRUDA was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Patients randomized to placebo and chemotherapy were offered KEYTRUDA as a single agent at the time of disease progression. Assessment of tumor status was performed at Week 6, Week 12, and then every 9 weeks thereafter. The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were ORR and DoR, as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 64 years (range: 34 to 84), 49% age 65 or older; 59% male; 94% White and 3% Asian; 56% ECOG PS of 1; and 18% with history of brain metastases. Thirty-one percent had tumor PD-L1 expression TPS <1% [negative]. Seventy-two percent received carboplatin and 12% were never smokers. A total of 85 patients in the placebo and chemotherapy arm received an anti-PD-1/PD-L1 monoclonal antibody at the time of disease progression.
The trial demonstrated a statistically significant improvement in OS and PFS for patients randomized to KEYTRUDA in combination with pemetrexed and platinum chemotherapy compared with placebo, pemetrexed, and platinum chemotherapy. Table 34 and Figure 4 summarize the efficacy results for KEYNOTE-189.
Table 34: Efficacy Results in KEYNOTE-189 Endpoint KEYTRUDA
200 mg every 3 weeks
Pemetrexed
Platinum Chemotherapy
n=410Placebo
Pemetrexed
Platinum Chemotherapy
n=206NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on stratified log-rank test.
- ‡ Response: Best objective response as confirmed complete response or partial response
- § Based on Miettinen and Nurminen method stratified by PD-L1 status, platinum chemotherapy and smoking status
OS Number (%) of patients with event 127 (31%) 108 (52%) Median in months (95% CI) NR
(NR, NR)11.3
(8.7, 15.1)Hazard ratio* (95% CI) 0.49 (0.38, 0.64) p-Value† <0.0001 PFS Number of patients with event (%) 244 (60%) 166 (81%) Median in months (95% CI) 8.8 (7.6, 9.2) 4.9 (4.7, 5.5) Hazard ratio* (95% CI) 0.52 (0.43, 0.64) p-Value† <0.0001 Objective Response Rate ORR‡ (95% CI) 48% (43, 53) 19% (14, 25) Complete response 0.5% 0.5% Partial response 47% 18% p-Value§ <0.0001 Duration of Response Median in months (range) 11.2 (1.1+, 18.0+) 7.8 (2.1+, 16.4+) Figure 4: Kaplan-Meier Curve for Overall Survival in KEYNOTE-189 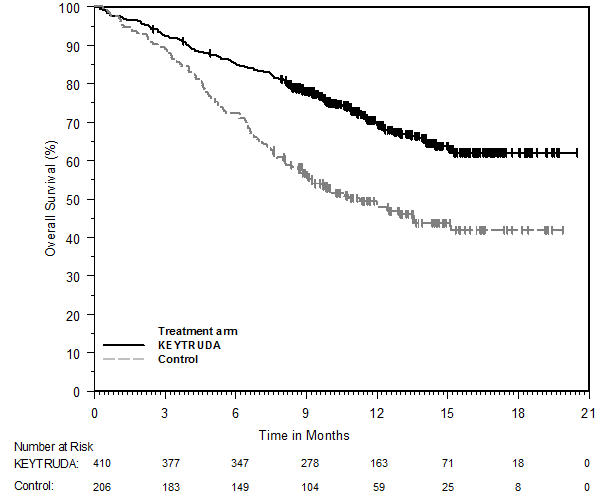
First-line treatment of metastatic squamous NSCLC with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy
The efficacy of KEYTRUDA in combination with carboplatin and investigator's choice of either paclitaxel or paclitaxel protein-bound was investigated in KEYNOTE-407 (NCT02775435), a randomized, multi-center, double-blind, placebo-controlled trial conducted in 559 patients with metastatic squamous NSCLC, regardless of PD-L1 tumor expression status, who had not previously received systemic therapy for metastatic disease. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by tumor PD-L1 status (TPS <1% [negative] vs. TPS ≥1%), choice of paclitaxel or paclitaxel protein-bound, and geographic region (East Asia vs. non-East Asia). Patients were randomized (1:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- KEYTRUDA 200 mg and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for 4 cycles, and paclitaxel 200 mg/m2 on Day 1 of each 21-day cycle for 4 cycles or paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 of each 21-day cycle for 4 cycles, followed by KEYTRUDA 200 mg every 3 weeks. KEYTRUDA was administered prior to chemotherapy on Day 1.
- Placebo and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for 4 cycles and paclitaxel 200 mg/m2 on Day 1 of each 21-day cycle for 4 cycles or paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 of each 21-day cycle for 4 cycles, followed by placebo every 3 weeks.
Treatment with KEYTRUDA and chemotherapy or placebo and chemotherapy continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Administration of KEYTRUDA was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Patients randomized to the placebo and chemotherapy arm were offered KEYTRUDA as a single agent at the time of disease progression. Assessment of tumor status was performed every 6 weeks through Week 18, every 9 weeks through Week 45 and every 12 weeks thereafter. The main efficacy outcome measures were PFS and ORR as assessed by BICR using RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS. An additional efficacy outcome measure was DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 65 years (range: 29 to 88), 55% age 65 or older; 81% male; 77% White; 71% ECOG PS of 1; and 8% with a history of brain metastases. Thirty-five percent had tumor PD-L1 expression TPS <1%; 19% were from the East Asian region; and 60% received paclitaxel.
The trial demonstrated a statistically significant improvement in OS, PFS and ORR in patients randomized to KEYTRUDA in combination with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy compared with patients randomized to placebo with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy. Table 35 and Figure 5 summarize the efficacy results for KEYNOTE-407.
Table 35: Efficacy Results in KEYNOTE-407 Endpoint KEYTRUDA
200 mg every 3 weeks
Carboplatin
Paclitaxel/Paclitaxel protein-bound
n=278Placebo
Carboplatin
Paclitaxel/Paclitaxel protein-bound
n=281NE = not estimable - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test
- ‡ ORR primary analysis and DoR analysis were conducted with the first 204 patients enrolled.
- § Based on a stratified Miettinen-Nurminen test
OS Number of events (%) 85 (31%) 120 (43%) Median in months (95% CI) 15.9 (13.2, NE) 11.3 (9.5, 14.8) Hazard ratio* (95% CI) 0.64 (0.49, 0.85) p-Value† 0.0017 PFS Number of events (%) 152 (55%) 197 (70%) Median in months (95% CI) 6.4 (6.2, 8.3) 4.8 (4.3, 5.7) Hazard ratio* (95% CI) 0.56 (0.45, 0.70) p-Value† <0.0001 n=101 n=103 Objective Response Rate‡ ORR (95% CI) 58% (48, 68) 35% (26, 45) Difference (95% CI) 23.6% (9.9, 36.4) p-Value§ 0.0008 Duration of Response‡ Median duration of response in months (range) 7.2 (2.4, 12.4+) 4.9 (2.0, 12.4+) Figure 5: Kaplan-Meier Curve for Overall Survival in KEYNOTE-407 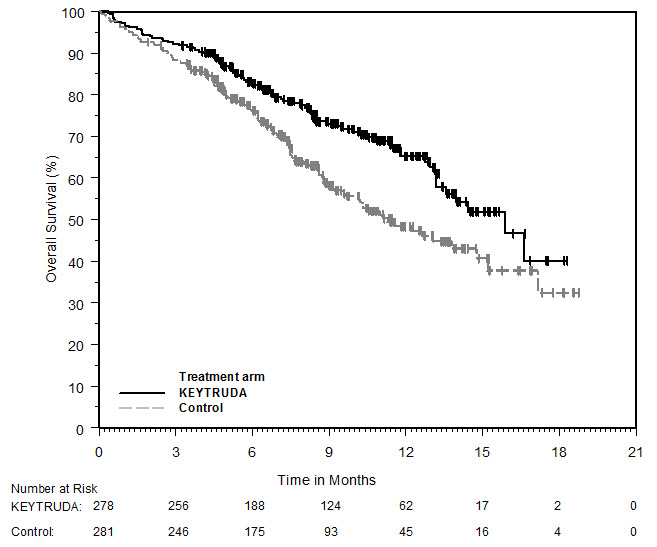
First-line treatment of metastatic NSCLC as a single agent
KEYNOTE-042
The efficacy of KEYTRUDA was investigated in KEYNOTE-042 (NCT02220894), a randomized, multicenter, open-label, active-controlled trial conducted in 1274 patients with stage III NSCLC who were not candidates for surgical resection or definitive chemoradiation, or patients with metastatic NSCLC. Only patients whose tumors expressed PD-L1 (TPS ≥1%) by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx kit and who had not received prior systemic treatment for metastatic NSCLC were eligible. Patients with EGFR or ALK genomic tumor aberrations; autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of radiation in the thoracic region within the prior 26 weeks of initiation of study were ineligible. Randomization was stratified by ECOG PS (0 vs. 1), histology (squamous vs. nonsquamous), geographic region (East Asia vs. non-East Asia), and PD-L1 expression (TPS ≥50% vs. TPS 1 to 49%). Patients were randomized (1:1) to receive KEYTRUDA 200 mg intravenously every 3 weeks or investigator's choice of either of the following platinum-containing chemotherapy regimens:
- Pemetrexed 500 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for a maximum of 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Paclitaxel 200 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for a maximum of 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies.
Treatment with KEYTRUDA continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease, unacceptable toxicity, or a maximum of 24 months. Administration of KEYTRUDA was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Treatment with KEYTRUDA could be reinitiated at the time of subsequent disease progression and administered for up to 12 months. Assessment of tumor status was performed every 9 weeks. The main efficacy outcome measure was OS in the subgroup of patients with TPS ≥50% NSCLC, the subgroup of patients with TPS ≥20% NSCLC, and the overall population with TPS ≥1% NSCLC. Additional efficacy outcome measures were PFS and ORR in the subgroup of patients with TPS ≥50% NSCLC, the subgroup of patients with TPS ≥20% NSCLC, and the overall population with TPS ≥1% NSCLC as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 63 years (range: 25 to 90), 45% age 65 or older; 71% male; and 64% White, 30% Asian, and 2% Black. Nineteen percent were Hispanic or Latino. Sixty-nine percent had ECOG PS of 1; 39% with squamous and 61% with nonsquamous histology; 87% had M1 disease and 13% had Stage IIIA (2%) or Stage IIIB (11%) and who were not candidates for surgical resection or definitive chemoradiation per investigator assessment; and 5% with treated brain metastases at baseline. Forty-seven percent of patients had TPS ≥50% NSCLC and 53% had TPS 1 to 49% NSCLC.
The trial demonstrated a statistically significant improvement in OS for patients (PD-L1 TPS ≥50%, TPS ≥20%, TPS ≥1%) randomized to KEYTRUDA as compared with chemotherapy. Table 36 and Figure 6 summarize the efficacy results in the subgroup of patients with TPS ≥50% and in all randomized patients with TPS ≥1%.
Table 36: Efficacy Results of All Randomized Patients (TPS ≥1% and TPS ≥50%) in KEYNOTE-042 TPS ≥1% TPS ≥50% Endpoint KEYTRUDA
200 mg every 3 weeksChemotherapy KEYTRUDA
200 mg every 3 weeksChemotherapy n=637 n=637 n=299 n=300 - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test; compared to a p-Value boundary of 0.0291
- ‡ Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints
- § Not significant compared to a p-Value boundary of 0.0291
- ¶ Based on observed duration of response
OS Number of events (%) 371 (58%) 438 (69%) 157 (53%) 199 (66%) Median in months (95% CI) 16.7 (13.9, 19.7) 12.1 (11.3, 13.3) 20.0 (15.4, 24.9) 12.2 (10.4, 14.2) Hazard ratio* (95% CI) 0.81 (0.71, 0.93) 0.69 (0.56, 0.85) p-Value† 0.0036 0.0006 PFS Number of events (%) 507 (80%) 506 (79%) 221 (74%) 233 (78%) Median in months (95% CI) 5.4 (4.3, 6.2) 6.5 (6.3, 7.0) 7.1 (5.9, 9.0) 6.4 (6.1, 6.9) Hazard ratio*, ‡ (95% CI) 1.07
(0.94, 1.21)0.81
(0.67, 0.99)p-Value† -‡ NS§ Objective Response Rate ORR‡ (95% CI) 27% (24, 31) 27% (23, 30) 39% (33.9, 45.3) 32% (26.8, 37.6) Complete response rate 0.5% 0.5% 0.7% 0.3% Partial response rate 27% 26% 39% 32% Duration of Response % with duration ≥12 months¶ 47% 16% 42% 17% % with duration ≥18 months¶ 26% 6% 25% 5% The results of all efficacy outcome measures in the subgroup of patients with PD-L1 TPS ≥20% NSCLC were intermediate between the results of those with PD-L1 TPS ≥1% and those with PD-L1 TPS ≥50%. In a pre-specified exploratory subgroup analysis for patients with TPS 1-49% NSCLC, the median OS was 13.4 months (95% CI: 10.7, 18.2) for the pembrolizumab group and 12.1 months (95% CI: 11.0, 14.0) in the chemotherapy group, with an HR of 0.92 (95% CI: 0.77, 1.11).
Figure 6: Kaplan-Meier Curve for Overall Survival in all Randomized Patients in KEYNOTE-042 (TPS ≥1%) 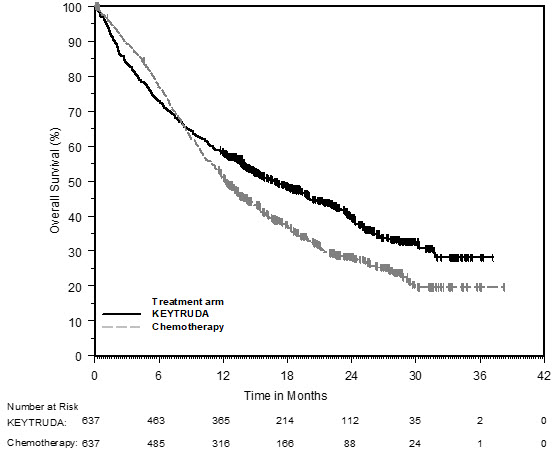
KEYNOTE-024
The efficacy of KEYTRUDA was also investigated in KEYNOTE-024 (NCT02142738), a randomized, multicenter, open-label, active-controlled trial in 305 previously untreated patients with metastatic NSCLC. The study design was similar to that of KEYNOTE-042, except that only patients whose tumors had high PD-L1 expression (TPS of 50% or greater) by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx kit were eligible. Patients were randomized (1:1) to receive KEYTRUDA 200 mg intravenously every 3 weeks or investigator's choice of any of the following platinum-containing chemotherapy regimens:
- Pemetrexed 500 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Pemetrexed 500 mg/m2 every 3 weeks and cisplatin 75 mg/m2 every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Gemcitabine 1250 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 every 3 weeks on Day 1 for 4 to 6 cycles;
- Gemcitabine 1250 mg/m2 on Days 1 and 8 and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles;
- Paclitaxel 200 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed maintenance (for nonsquamous histologies).
Patients randomized to chemotherapy were offered KEYTRUDA at the time of disease progression.
The main efficacy outcome measure was PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were OS and ORR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 65 years (range: 33 to 90), 54% age 65 or older; 61% male; 82% White and 15% Asian; 65% with ECOG PS of 1; 18% with squamous and 82% with nonsquamous histology and 9% with history of brain metastases. A total of 66 patients in the chemotherapy arm received KEYTRUDA at the time of disease progression.
The trial demonstrated a statistically significant improvement in both PFS and OS for patients randomized to KEYTRUDA as compared with chemotherapy. Table 37 and Figure 7 summarize the efficacy results for KEYNOTE-024.
Table 37: Efficacy Results in KEYNOTE-024 Endpoint KEYTRUDA
200 mg every 3 weeksChemotherapy n=154 n=151 NR = not reached - * Based on the stratified Cox proportional hazard model for the interim analysis
- † Based on the protocol-specified final OS analysis conducted at 169 events, which occurred 14 months after the interim analysis.
- ‡ p-Value is compared with 0.0118 of the allocated alpha for the interim analysis
PFS Number (%) of patients with event 73 (47%) 116 (77%) Median in months (95% CI) 10.3 (6.7, NR) 6.0 (4.2, 6.2) Hazard ratio* (95% CI) 0.50 (0.37, 0.68) p-Value (stratified log-rank) <0.001 OS Number (%) of patients with event 44 (29%) 64 (42%) Median in months (95% CI)† 30.0
(18.3, NR)14.2
(9.8, 19.0)Hazard ratio* (95% CI) 0.60 (0.41, 0.89) p-Value (stratified log-rank) 0.005‡ Objective Response Rate ORR (95% CI) 45% (37, 53) 28% (21, 36) Complete response rate 4% 1% Partial response rate 41% 27% p-Value (Miettinen-Nurminen) 0.001 Median duration of response in months (range) NR
(1.9+, 14.5+)6.3
(2.1+, 12.6+)Previously treated NSCLC
The efficacy of KEYTRUDA was investigated in KEYNOTE-010 (NCT01905657), a randomized, multicenter, open-label, active-controlled trial conducted in 1033 patients with metastatic NSCLC that had progressed following platinum-containing chemotherapy, and if appropriate, targeted therapy for EGFR or ALK genomic tumor aberrations. Eligible patients had PD-L1 expression TPS of 1% or greater by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx kit. Patients with autoimmune disease; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by tumor PD-L1 expression (PD-L1 expression TPS ≥50% vs. PD-L1 expression TPS=1-49%), ECOG PS (0 vs. 1), and geographic region (East Asia vs. non-East Asia). Patients were randomized (1:1:1) to receive KEYTRUDA 2 mg/kg intravenously every 3 weeks, KEYTRUDA 10 mg/kg intravenously every 3 weeks or docetaxel intravenously 75 mg/m2 every 3 weeks until unacceptable toxicity or disease progression. Patients randomized to KEYTRUDA were permitted to continue until disease progression that was symptomatic, rapidly progressive, required urgent intervention, occurred with a decline in performance status, or confirmation of progression at 4 to 6 weeks with repeat imaging or for up to 24 months without disease progression. Assessment of tumor status was performed every 9 weeks. The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, in the subgroup of patients with TPS ≥50% and the overall population with TPS ≥1%. Additional efficacy outcome measures were ORR and DoR in the subgroup of patients with TPS ≥50% and the overall population with TPS ≥1%.
The study population characteristics were: median age of 63 years (range: 20 to 88), 42% age 65 or older; 61% male; 72% White and 21% Asian; 66% ECOG PS of 1; 43% with high PD-L1 tumor expression; 21% with squamous, 70% with nonsquamous, and 8% with mixed, other or unknown histology; 91% metastatic (M1) disease; 15% with history of brain metastases; and 8% and 1% with EGFR and ALK genomic aberrations, respectively. All patients had received prior therapy with a platinum-doublet regimen, 29% received two or more prior therapies for their metastatic disease.
Tables 38 and 39 and Figure 8 summarize efficacy results in the subgroup with TPS ≥50% population and in all patients, respectively.
Table 38: Efficacy Results of the Subgroup of Patients with TPS ≥50% in KEYNOTE-010 Endpoint KEYTRUDA
2 mg/kg every 3 weeks
n=139KEYTRUDA
10 mg/kg every 3 weeks
n=151Docetaxel
75 mg/m2 every 3 weeks
n=152NR = not reached - * Hazard ratio (KEYTRUDA compared to docetaxel) based on the stratified Cox proportional hazard model
- † All responses were partial responses
OS Deaths (%) 58 (42%) 60 (40%) 86 (57%) Median in months (95% CI) 14.9 (10.4, NR) 17.3 (11.8, NR) 8.2 (6.4, 10.7) Hazard ratio* (95% CI) 0.54 (0.38, 0.77) 0.50 (0.36, 0.70) --- p-Value (stratified log-rank) <0.001 <0.001 --- PFS Events (%) 89 (64%) 97 (64%) 118 (78%) Median in months (95% CI) 5.2 (4.0, 6.5) 5.2 (4.1, 8.1) 4.1 (3.6, 4.3) Hazard ratio* (95% CI) 0.58 (0.43, 0.77) 0.59 (0.45, 0.78) --- p-Value (stratified log-rank) <0.001 <0.001 --- Objective Response Rate ORR† (95% CI) 30% (23, 39) 29% (22, 37) 8% (4, 13) p-Value (Miettinen-Nurminen) <0.001 <0.001 --- Median duration of response in months (range) NR
(0.7+, 16.8+)NR
(2.1+, 17.8+)8.1
(2.1+, 8.8+)Table 39: Efficacy Results of All Randomized Patients (TPS ≥1%) in KEYNOTE-010 Endpoint KEYTRUDA
2 mg/kg every 3 weeks
n=344KEYTRUDA
10 mg/kg every 3 weeks
n=346Docetaxel
75 mg/m2 every 3 weeks
n=343NR = not reached - * Hazard ratio (KEYTRUDA compared to docetaxel) based on the stratified Cox proportional hazard model
- † All responses were partial responses
OS Deaths (%) 172 (50%) 156 (45%) 193 (56%) Median in months (95% CI) 10.4 (9.4, 11.9) 12.7 (10.0, 17.3) 8.5 (7.5, 9.8) Hazard ratio* (95% CI) 0.71 (0.58, 0.88) 0.61 (0.49, 0.75) --- p-Value (stratified log-rank) <0.001 <0.001 --- PFS Events (%) 266 (77%) 255 (74%) 257 (75%) Median in months (95% CI) 3.9 (3.1, 4.1) 4.0 (2.6, 4.3) 4.0 (3.1, 4.2) Hazard ratio* (95% CI) 0.88 (0.73, 1.04) 0.79 (0.66, 0.94) --- p-Value (stratified log-rank) 0.068 0.005 --- Objective Response Rate ORR† (95% CI) 18% (14, 23) 19% (15, 23) 9% (7, 13) p-Value (Miettinen-Nurminen) <0.001 <0.001 --- Median duration of response in months (range) NR
(0.7+, 20.1+)NR
(2.1+, 17.8+)6.2
(1.4+, 8.8+)Figure 8: Kaplan-Meier Curve for Overall Survival in all Randomized Patients in KEYNOTE-010 (TPS ≥1%) 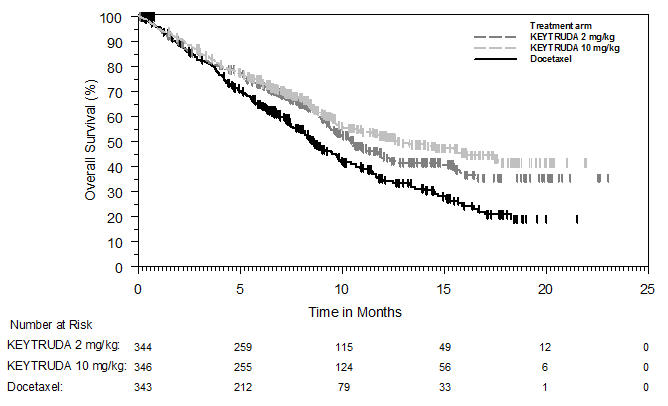
14.3 Small Cell Lung Cancer
The efficacy of KEYTRUDA was investigated in 83 patients with SCLC who had disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy enrolled in one of two multicenter, multi-cohort, non-randomized, open label trials: KEYNOTE-028 (NCT02054806), Cohort C1, or KEYNOTE-158 (NCT02628067), Cohort G. The trials excluded patients with autoimmune disease or a medical condition that required immunosuppression.
Patients received either KEYTRUDA 200 mg intravenously every 3 weeks (n=64) or 10 mg/kg intravenously every 2 weeks (n=19). Treatment with KEYTRUDA continued until documented disease progression, unacceptable toxicity, or a maximum of 24 months. Patients with initial radiographic disease progression could receive additional doses of KEYTRUDA during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status.
Assessment of tumor status was performed every 8 weeks for the first 6 months in KEYNOTE-028, every 9 weeks for the first 12 months in KEYNOTE-158, and every 12 weeks thereafter for both studies. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 62 years (range: 24 to 84); 40% age 65 or older; 64% male; 63% White, 25% Asian, and 2% Black; 30% ECOG PS of 0 and 69% ECOG PS of 1; 7% had M0 disease and 93% had M1 disease; and 16% had a history of brain metastases. Sixty-four percent received two prior lines of therapy and 36% received three or more lines of therapy; 60% received prior thoracic radiation therapy; 51% received prior radiation therapy to the brain.
Efficacy results are summarized in Table 40.
Table 40: Efficacy Results in Patients with Small Cell Lung Cancer Endpoint KEYTRUDA
n=83- * Denotes ongoing response
Objective Response Rate ORR (95% CI) 19% (11, 29) Complete response rate 2% Partial response rate 17% Duration of Response n=16 Range (months) 4.1, 35.8* % with duration ≥6 months 94% % with duration ≥12 months 63% % with duration ≥18 months 56% 14.4 Head and Neck Squamous Cell Cancer
First-line treatment of metastatic or unresectable, recurrent HNSCC
The efficacy of KEYTRUDA was investigated in KEYNOTE-048 (NCT02358031), a randomized, multicenter, open-label, active-controlled trial conducted in 882 patients with metastatic HNSCC who had not previously received systemic therapy for metastatic disease or with recurrent disease who were considered incurable by local therapies. Patients with active autoimmune disease that required systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by tumor PD-L1 expression (TPS ≥50% or <50%) according to the PD-L1 IHC 22C3 pharmDx kit, HPV status according to p16 IHC (positive or negative), and ECOG PS (0 vs. 1). Patients were randomized 1:1:1 to one of the following treatment arms:
- KEYTRUDA 200 mg intravenously every 3 weeks
- KEYTRUDA 200 mg intravenously every 3 weeks, carboplatin AUC 5 mg/mL/min intravenously every 3 weeks or cisplatin 100 mg/m2 intravenously every 3 weeks, and FU 1000 mg/m2/day as a continuous intravenous infusion over 96 hours every 3 weeks (maximum of 6 cycles of platinum and FU)
- Cetuximab 400 mg/m2 intravenously as the initial dose then 250 mg/m2 intravenously once weekly, carboplatin AUC 5 mg/mL/min intravenously every 3 weeks or cisplatin 100 mg/m2 intravenously every 3 weeks, and FU 1000 mg/m2/day as a continuous intravenous infusion over 96 hours every 3 weeks (maximum of 6 cycles of platinum and FU)
Treatment with KEYTRUDA continued until RECIST v1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of KEYTRUDA was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at Week 9 and then every 6 weeks for the first year, followed by every 9 weeks through 24 months. A retrospective re-classification of patients' tumor PD-L1 status according to CPS using the PD-L1 IHC 22C3 pharmDx kit was conducted using the tumor specimens used for randomization.
The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ) sequentially tested in the subgroup of patients with CPS ≥20, the subgroup of patients with CPS ≥1, and the overall population.
The study population characteristics were: median age of 61 years (range: 20 to 94), 36% age 65 or older; 83% male; 73% White, 20% Asian and 2.4% Black; 61% had ECOG PS of 1; and 79% were former/current smokers. Twenty-two percent of patients' tumors were HPV-positive, 23% had PD-L1 TPS ≥50%, and 95% had Stage IV disease (Stage IVA 19%, Stage IVB 6%, and Stage IVC 70%). Eighty-five percent of patients' tumors had PD-L1 expression of CPS ≥1 and 43% had CPS ≥20.
The trial demonstrated a statistically significant improvement in OS for patients randomized to KEYTRUDA in combination with chemotherapy compared to those randomized to cetuximab in combination with chemotherapy at a pre-specified interim analysis in the overall population. The trial also demonstrated a statistically significant improvement in OS for the subgroup of patients with PD-L1 CPS ≥1 randomized to KEYTRUDA as a single agent compared to those randomized to cetuximab in combination with chemotherapy. At the time of the interim analysis, there was no significant difference in OS between the KEYTRUDA single agent arm and the control arm for the overall population. Table 41 and Figure 9 summarize efficacy results for KEYTRUDA in combination with chemotherapy.
Table 41: Efficacy Results for KEYTRUDA plus Platinum/Fluorouracil in KEYNOTE-048 Endpoint KEYTRUDA
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FUn=281 n=278 - * Based on the stratified Cox proportional hazard model
- † Based on stratified log-rank test
- ‡ Response: Best objective response as confirmed complete response or partial response
OS Number (%) of patients with event 197 (70%) 223 (80%) Median in months (95% CI) 13.0 (10.9, 14.7) 10.7 (9.3, 11.7) Hazard ratio* (95% CI) 0.77 (0.63, 0.93) p-Value† 0.0067 PFS Number of patients with event (%) 244 (87%) 253 (91%) Median in months (95% CI) 4.9 (4.7, 6.0) 5.1 (4.9, 6.0) Hazard ratio* (95% CI) 0.92 (0.77, 1.10) p-Value† 0.3394 Objective Response Rate ORR‡ (95% CI) 36% (30.0, 41.5) 36% (30.7, 42.3) Complete response rate 6% 3% Partial response rate 30% 33% Duration of Response Median in months (range) 6.7 (1.6+, 30.4+) 4.3 (1.2+, 27.9+) In KEYNOTE-048, OS HRs for patients randomized to KEYTRUDA in combination with chemotherapy, compared with cetuximab in combination with chemotherapy, were similar for all populations regardless of PD-L1 expression in a pre-specified interim analysis: ITT (HR 0.77, 95% CI: 0.63, 0.93), CPS ≥1 (HR 0.71, 95% CI: 0.57, 0.88), CPS ≥20 (HR 0.69, 95% CI:0.51, 0.94).
Figure 9: Kaplan-Meier Curve for Overall Survival for KEYTRUDA plus Platinum/Fluorouracil in KEYNOTE-048 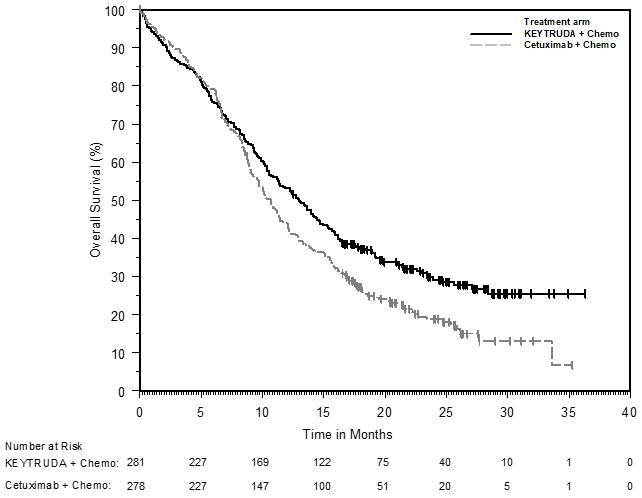
Table 42 summarizes efficacy results for KEYTRUDA as a single agent in the subgroups of patients with CPS ≥1 HNSCC and CPS ≥20 HNSCC. Figure 10 summarizes the OS results in the subgroup of patients with CPS ≥1 HNSCC.
Table 42: Efficacy Results for KEYTRUDA as a Single Agent in KEYNOTE-048 (CPS ≥1 and CPS ≥20) Endpoint CPS ≥1 CPS ≥20 KEYTRUDA
200 mg every 3 weeksCetuximab
Platinum
FUKEYTRUDA
200 mg every 3 weeksCetuximab
Platinum
FUn=257 n=255 n=133 n=122 - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test
- ‡ Response: Best objective response as confirmed complete response or partial response
OS Number of events (%) 177 (69%) 206 (81%) 82 (62%) 95 (78%) Median in months (95% CI) 12.3 (10.8, 14.9) 10.3 (9.0,11.5) 14.9 (11.6, 21.5) 10.7 (8.8, 12.8) Hazard ratio* (95% CI) 0.78 (0.64, 0.96) 0.61 (0.45, 0.83) p-Value† 0.0171 0.0015 PFS Number of events (%) 225 (88%) 231 (91%) 113 (85%) 111 (91%) Median in months (95% CI) 3.2 (2.2, 3.4) 5.0 (4.8, 5.8) 3.4 (3.2, 3.8) 5.0 (4.8, 6.2) Hazard ratio * (95% CI) 1.15(0.95, 1.38) 0.99 (0.75, 1.29) Objective Response Rate ORR‡ (95% CI) 19% (14.5, 24.4) 35% (29.1, 41.1) 23% (16.4, 31.4) 36% (27.6, 45.3) Complete response rate 5% 3% 8% 3% Partial response rate 14% 32% 16% 33% Duration of Response Median in months (range) 20.9 (1.5+, 34.8+) 4.5 (1.2+, 28.6+) 20.9 (2.7, 34.8+) 4.2 (1.2+, 22.3+) In an exploratory subgroup analysis for patients with CPS 1-19 HNSCC, the median OS was 10.8 months (95% CI: 9.0, 12.6) for KEYTRUDA as a single agent and 10.1 months (95% CI: 8.7, 12.1) for cetuximab in combination with chemotherapy, with an HR of 0.90 (95% CI: 0.68, 1.18).
Figure 10: Kaplan-Meier Curve for Overall Survival for KEYTRUDA as a Single Agent in KEYNOTE-048 (CPS ≥1) 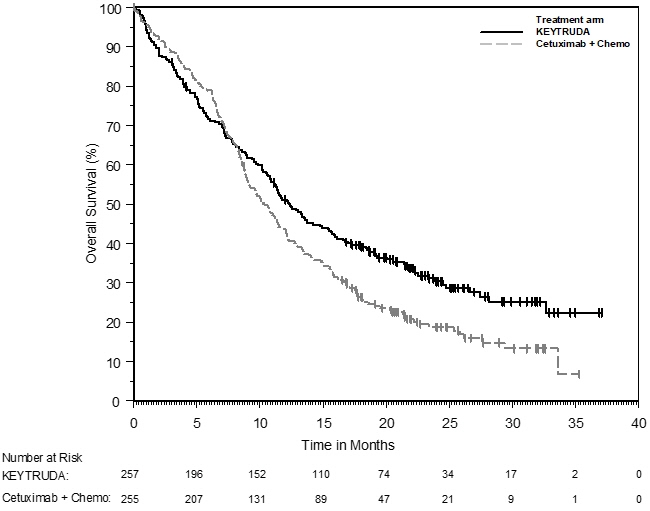
Previously treated recurrent or metastatic HNSCC
The efficacy of KEYTRUDA was investigated in KEYNOTE-012 (NCT01848834), a multicenter, non-randomized, open-label, multi-cohort study that enrolled 174 patients with recurrent or metastatic HNSCC who had disease progression on or after platinum-containing chemotherapy administered for recurrent or metastatic HNSCC or following platinum-containing chemotherapy administered as part of induction, concurrent, or adjuvant therapy. Patients with active autoimmune disease, a medical condition that required immunosuppression, evidence of interstitial lung disease, or ECOG PS ≥2 were ineligible.
Patients received KEYTRUDA 10 mg/kg every 2 weeks (n=53) or 200 mg every 3 weeks (n=121) until unacceptable toxicity or disease progression that was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at least 4 weeks later with repeat imaging. Patients without disease progression were treated for up to 24 months. Treatment with pembrolizumab could be reinitiated for subsequent disease progression and administered for up to 1 additional year. Assessment of tumor status was performed every 8 weeks. The major efficacy outcome measures were ORR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR, and DoR.
The study population characteristics were median age of 60 years, 32% age 65 or older; 82% male; 75% White, 16% Asian, and 6% Black; 87% had M1 disease; 33% had HPV positive tumors; 63% had prior cetuximab; 29% had an ECOG PS of 0 and 71% had an ECOG PS of 1; and the median number of prior lines of therapy administered for the treatment of HNSCC was 2.
The ORR was 16% (95% CI: 11, 22) with a complete response rate of 5%. The median follow-up time was 8.9 months. Among the 28 responding patients, the median DoR had not been reached (range: 2.4+ to 27.7+ months), with 23 patients having responses of 6 months or longer. The ORR and DoR were similar irrespective of dosage regimen (10 mg/kg every 2 weeks or 200 mg every 3 weeks) or HPV status.
14.5 Classical Hodgkin Lymphoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-087 (NCT02453594), a multicenter, non-randomized, open-label trial in 210 patients with relapsed or refractory cHL. Patients with active, non-infectious pneumonitis, an allogeneic HSCT within the past 5 years (or > 5 years but with symptoms of GVHD), active autoimmune disease, a medical condition that required immunosuppression, or an active infection requiring systemic therapy were ineligible for the trial. Patients received KEYTRUDA 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress. Disease assessment was performed every 12 weeks. The major efficacy outcome measures (ORR, Complete Response Rate, and DoR) were assessed by BICR according to the 2007 revised International Working Group (IWG) criteria.
The study population characteristics were: median age of 35 years (range: 18 to 76), 9% age 65 or older; 54% male; 88% White; and 49% ECOG PS of 0 and 51% ECOG PS of 1. The median number of prior lines of therapy administered for the treatment of cHL was 4 (range: 1 to 12). Fifty-eight percent were refractory to the last prior therapy, including 35% with primary refractory disease and 14% whose disease was chemo-refractory to all prior regimens. Sixty-one percent of patients had undergone prior auto-HSCT, 83% had received prior brentuximab vedotin and 36% of patients had prior radiation therapy.
Efficacy results for KEYNOTE-087 are summarized in Table 43.
Table 43: Efficacy Results in KEYNOTE-087 Endpoint KEYTRUDA
200 mg every 3 weeks
n=210*- * Median follow-up time of 9.4 months
- † Based on patients (n=145) with a response by independent review
Objective Response Rate ORR (95% CI) 69% (62, 75) Complete response rate 22% Partial response rate 47% Duration of Response Median in months (range) 11.1 (0.0+, 11.1) † 14.6 Primary Mediastinal Large B-Cell Lymphoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-170 (NCT02576990), a multicenter, open-label, single-arm trial in 53 patients with relapsed or refractory PMBCL. Patients were not eligible if they had active non-infectious pneumonitis, allogeneic HSCT within the past 5 years (or >5 years but with symptoms of GVHD), active autoimmune disease, a medical condition that required immunosuppression, or an active infection requiring systemic therapy. Patients were treated with KEYTRUDA 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months for patients who did not progress. Disease assessments were performed every 12 weeks and assessed by BICR according to the 2007 revised IWG criteria. The efficacy outcome measures were ORR and DoR.
The study population characteristics were: median age of 33 years (range: 20 to 61 years); 43% male; 92% White; and 43% ECOG PS of 0 and 57% ECOG PS of 1. The median number of prior lines of therapy administered for the treatment of PMBCL was 3 (range 2 to 8). Thirty-six percent had primary refractory disease, 49% had relapsed disease refractory to the last prior therapy, and 15% had untreated relapse. Twenty-six percent of patients had undergone prior autologous HSCT, and 32% of patients had prior radiation therapy. All patients had received rituximab as part of a prior line of therapy.
For the 24 responders, the median time to first objective response (complete or partial response) was 2.8 months (range 2.1 to 8.5 months). Efficacy results for KEYNOTE-170 are summarized in Table 44.
Table 44: Efficacy Results in KEYNOTE-170 Endpoint KEYTRUDA
200 mg every 3 weeks
n=53*NR = not reached - * Median follow-up time of 9.7 months
- † Based on patients (n=24) with a response by independent review
Objective Response Rate ORR (95% CI) 45% (32, 60) Complete response rate 11% Partial response rate 34% Duration of Response Median in months (range) NR (1.1+, 19.2+)† 14.7 Urothelial Carcinoma
Cisplatin Ineligible Patients with Urothelial Carcinoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-052 (NCT02335424), a multicenter, open-label, single-arm trial in 370 patients with locally advanced or metastatic urothelial carcinoma who were not eligible for cisplatin-containing chemotherapy. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression. Patients received KEYTRUDA 200 mg every 3 weeks until unacceptable toxicity or disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Tumor response assessments were performed at 9 weeks after the first dose, then every 6 weeks for the first year, and then every 12 weeks thereafter. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 74 years; 77% male; and 89% White. Eighty-seven percent had M1 disease, and 13% had M0 disease. Eighty-one percent had a primary tumor in the lower tract, and 19% of patients had a primary tumor in the upper tract. Eighty-five percent of patients had visceral metastases, including 21% with liver metastases. Reasons for cisplatin ineligibility included: 50% with baseline creatinine clearance of <60 mL/min, 32% with ECOG PS of 2, 9% with ECOG PS of 2 and baseline creatinine clearance of <60 mL/min, and 9% with other reasons (Class III heart failure, Grade 2 or greater peripheral neuropathy, and Grade 2 or greater hearing loss). Ninety percent of patients were treatment naïve, and 10% received prior adjuvant or neoadjuvant platinum-based chemotherapy.
Among the 370 patients, 30% (n = 110) had tumors that expressed PD-L1 with a CPS ≥10. PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx kit. The study population characteristics of these 110 patients were: median age of 73 years; 68% male; and 87% White. Eighty-two percent had M1 disease, and 18% had M0 disease. Eighty-one percent had a primary tumor in the lower tract, and 18% of patients had a primary tumor in the upper tract. Seventy-six percent of patients had visceral metastases, including 11% with liver metastases. Reasons for cisplatin ineligibility included: 45% with baseline creatinine clearance of <60 mL/min, 37% with ECOG PS of 2, 10% with ECOG PS of 2 and baseline creatinine clearance of <60 mL/min, and 8% with other reasons (Class III heart failure, Grade 2 or greater peripheral neuropathy, and Grade 2 or greater hearing loss). Ninety percent of patients were treatment naïve, and 10% received prior adjuvant or neoadjuvant platinum-based chemotherapy.
The median follow-up time for 370 patients treated with KEYTRUDA was 7.8 months (range 0.1 to 20 months). Efficacy results are summarized in Table 45.
Table 45: Efficacy Results in KEYNOTE-052 Endpoint KEYTRUDA
200 mg every 3 weeksAll Subjects
n=370PD-L1 CPS <10
n=260*PD-L1 CPS ≥10
n=110+ Denotes ongoing
NR = not reached- * Includes 9 subjects with unknown PD-L1 status
Objective Response Rate ORR (95% CI) 29% (24, 34) 21% (16, 26) 47% (38, 57) Complete response rate 7% 3% 15% Partial response rate 22% 18% 32% Duration of Response Median in months (range) NR
(1.4+, 17.8+)NR
(1.4+, 16.3+)NR
(1.4+, 17.8+)Previously Untreated Urothelial Carcinoma
KEYNOTE-361 (NCT02853305) is an ongoing, multicenter, randomized study in previously untreated patients with metastatic urothelial carcinoma who are eligible for platinum-containing chemotherapy. The study compares KEYTRUDA with or without platinum-based chemotherapy (i.e., cisplatin or carboplatin with gemcitabine) to platinum-based chemotherapy alone. The trial also enrolled a third arm of monotherapy with KEYTRUDA to compare to platinum-based chemotherapy alone. The independent Data Monitoring Committee (iDMC) for the study conducted a review of early data and found that in patients classified as having low PD-L1 expression (CPS <10), those treated with KEYTRUDA monotherapy had decreased survival compared to those who received platinum-based chemotherapy. The iDMC recommended to stop further accrual of patients with low PD-L1 expression in the monotherapy arm, however, no other changes were recommended, including any change of therapy for patients who had already been randomized to and were receiving treatment in the monotherapy arm.
Previously Treated Urothelial Carcinoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-045 (NCT02256436), a multicenter, randomized (1:1), active-controlled trial in 542 patients with locally advanced or metastatic urothelial carcinoma with disease progression on or after platinum-containing chemotherapy. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression.
Patients were randomized to receive either KEYTRUDA 200 mg every 3 weeks (n=270) or investigator's choice of any of the following chemotherapy regimens all given intravenously every 3 weeks (n=272): paclitaxel 175 mg/m2 (n=90), docetaxel 75 mg/m2 (n=92), or vinflunine 320 mg/m2 (n=90). Treatment continued until unacceptable toxicity or disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Assessment of tumor status was performed at 9 weeks after randomization, then every 6 weeks through the first year, followed by every 12 weeks thereafter. The major efficacy outcomes were OS and PFS as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were ORR as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and DoR.
The study population characteristics were: median age of 66 years (range: 26 to 88), 58% age 65 or older; 74% male; 72% White and 23% Asian; 42% ECOG PS of 0 and 56% ECOG PS of 1; and 96% M1 disease and 4% M0 disease. Eighty-seven percent of patients had visceral metastases, including 34% with liver metastases. Eighty-six percent had a primary tumor in the lower tract and 14% had a primary tumor in the upper tract. Fifteen percent of patients had disease progression following prior platinum-containing neoadjuvant or adjuvant chemotherapy. Twenty-one percent had received 2 or more prior systemic regimens in the metastatic setting. Seventy-six percent of patients received prior cisplatin, 23% had prior carboplatin, and 1% were treated with other platinum-based regimens.
The study demonstrated statistically significant improvements in OS and ORR for patients randomized to KEYTRUDA as compared to chemotherapy. There was no statistically significant difference between KEYTRUDA and chemotherapy with respect to PFS. The median follow-up time for this trial was 9.0 months (range: 0.2 to 20.8 months). Table 46 and Figure 11 summarize the efficacy results for KEYNOTE-045.
Table 46: Efficacy Results in KEYNOTE-045 KEYTRUDA
200 mg every 3 weeksChemotherapy n=270 n=272 + Denotes ongoing
NR = not reached- * Hazard ratio (KEYTRUDA compared to chemotherapy) based on the stratified Cox proportional hazard model
OS Deaths (%) 155 (57%) 179 (66%) Median in months (95% CI) 10.3 (8.0, 11.8) 7.4 (6.1, 8.3) Hazard ratio* (95% CI) 0.73 (0.59, 0.91) p-Value (stratified log-rank) 0.004 PFS by BICR Events (%) 218 (81%) 219 (81%) Median in months (95% CI) 2.1 (2.0, 2.2) 3.3 (2.3, 3.5) Hazard ratio* (95% CI) 0.98 (0.81, 1.19) p-Value (stratified log-rank) 0.833 Objective Response Rate ORR (95% CI) 21% (16, 27) 11% (8, 16) Complete response rate 7% 3% Partial response rate 14% 8% p-Value (Miettinen-Nurminen) 0.002 Median duration of response in months (range) NR
(1.6+, 15.6+)4.3
(1.4+, 15.4+)Figure 11: Kaplan-Meier Curve for Overall Survival in KEYNOTE-045 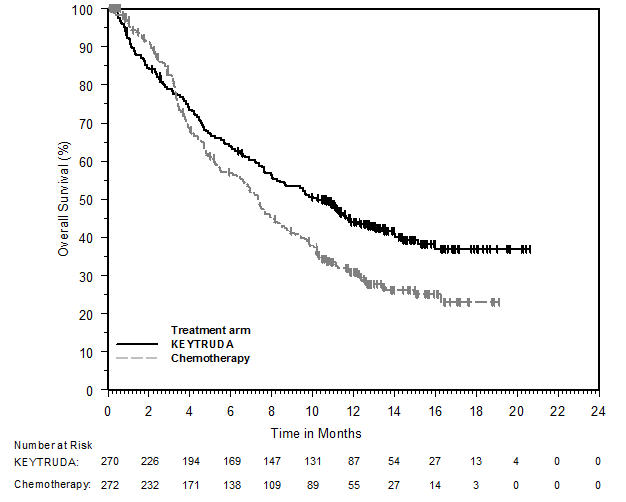
BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer
The efficacy of KEYTRUDA was investigated in KEYNOTE-057 (NCT02625961), a multicenter, open-label, single-arm trial in 96 patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. BCG-unresponsive high-risk NMIBC was defined as persistent disease despite adequate BCG therapy, disease recurrence after an initial tumor-free state following adequate BCG therapy, or T1 disease following a single induction course of BCG. Adequate BCG therapy was defined as administration of at least five of six doses of an initial induction course plus either of: at least two of three doses of maintenance therapy or at least two of six doses of a second induction course. Prior to treatment, all patients had undergone transurethral resection of bladder tumor (TURBT) to remove all resectable disease (Ta and T1 components). Residual CIS (Tis components) not amenable to complete resection was allowed. The trial excluded patients with muscle invasive (i.e., T2, T3, T4) locally advanced non-resectable or metastatic urothelial carcinoma, concurrent extra-vesical (i.e., urethra, ureter or renal pelvis) non-muscle invasive transitional cell carcinoma of the urothelium, or autoimmune disease or a medical condition that required immunosuppression.
Patients received KEYTRUDA 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or progressive disease. Assessment of tumor status was performed every 12 weeks for two years and then every 24 weeks for three years, and patients without disease progression could be treated for up to 24 months. The major efficacy outcome measures were complete response (as defined by negative results for cystoscopy [with TURBT/biopsies as applicable], urine cytology, and computed tomography urography [CTU] imaging) and duration of response.
The study population characteristics were: median age of 73 years (range: 44 to 92); 44% age ≥75; 84% male; 67% White; and 73% and 27% with an ECOG performance status of 0 or 1, respectively. Tumor pattern at study entry was CIS with T1 (13%), CIS with high grade TA (25%), and CIS (63%). Baseline high-risk NMIBC disease status was 27% persistent and 73% recurrent. The median number of prior instillations of BCG was 12.
The median follow-up time was 28.0 months (range: 4.6 to 40.5 months). Efficacy results are summarized in Table 47.
Table 47: Efficacy Results in KEYNOTE-057 Endpoint KEYTRUDA
200 mg every 3 weeks
n=96- * Based on patients (n=39) that achieved a complete response; reflects period from the time complete response was achieved
- † Denotes ongoing
Complete Response Rate (95% CI) 41% (31, 51) Duration of Response* Median in months (range) 16.2 (0.0+, 30.4†) % (n) with duration ≥12 months 46% (18) 14.8 Microsatellite Instability-High Cancer
The efficacy of KEYTRUDA was investigated in patients with MSI-H or mismatch repair deficient (dMMR), solid tumors enrolled in one of five uncontrolled, open-label, multi-cohort, multi-center, single-arm trials. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible across the five trials. Patients received either KEYTRUDA 200 mg every 3 weeks or KEYTRUDA 10 mg/kg every 2 weeks. Treatment continued until unacceptable toxicity or disease progression that was either symptomatic, rapidly progressive, required urgent intervention, or occurred with a decline in performance status. A maximum of 24 months of treatment with KEYTRUDA was administered. For the purpose of assessment of anti-tumor activity across these 5 trials, the major efficacy outcome measures were ORR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and DoR.
Table 48: MSI-H Trials Study Design and Patient Population Number of Patients MSI-H/dMMR Testing Dosage Prior Therapy CRC = colorectal cancer
PCR = polymerase chain reaction
IHC = immunohistochemistryKEYNOTE-016
NCT01876511- prospective, investigator-initiated
- 6 sites
- patients with CRC and other tumors
28 CRC
30 non-CRClocal PCR or IHC 10 mg/kg every 2 weeks - CRC: ≥ 2 prior regimens
- Non-CRC: ≥1 prior regimen
KEYNOTE-164
NCT02460198- prospective international multi-center
- CRC
61 local PCR or IHC 200 mg every 3 weeks Prior fluoropyrimidine, oxaliplatin, and irinotecan +/- anti-VEGF/EGFR mAb KEYNOTE-012
NCT01848834- retrospectively identified patients with PD-L1-positive gastric, bladder, or triple-negative breast cancer
6 central PCR 10 mg/kg every 2 weeks ≥1 prior regimen KEYNOTE-028
NCT02054806- retrospectively identified patients with PD-L1-positive esophageal, biliary, breast, endometrial, or CRC
5 central PCR 10 mg/kg every 2 weeks ≥1 prior regimen KEYNOTE-158
NCT02628067- prospective international multi-center enrollment of patients with MSI-H/dMMR non-CRC
- retrospectively identified patients who were enrolled in specific rare tumor non-CRC cohorts
19 local PCR or IHC (central PCR for patients in rare tumor non-CRC cohorts) 200 mg every 3 weeks ≥1 prior regimen Total 149 A total of 149 patients with MSI-H or dMMR cancers were identified across the five trials. Among these 149 patients, the baseline characteristics were: median age of 55 years, 36% age 65 or older; 56% male; 77% White, 19% Asian, and 2% Black; and 36% ECOG PS of 0 and 64% ECOG PS of 1. Ninety-eight percent of patients had metastatic disease and 2% had locally advanced, unresectable disease. The median number of prior therapies for metastatic or unresectable disease was two. Eighty-four percent of patients with metastatic CRC and 53% of patients with other solid tumors received two or more prior lines of therapy.
The identification of MSI-H or dMMR tumor status for the majority of patients (135/149) was prospectively determined using local laboratory-developed, polymerase chain reaction (PCR) tests for MSI-H status or immunohistochemistry (IHC) tests for dMMR. Fourteen of the 149 patients were retrospectively identified as MSI-H by testing tumor samples from a total of 415 patients using a central laboratory developed PCR test. Forty-seven patients had dMMR cancer identified by IHC, 60 had MSI-H identified by PCR, and 42 were identified using both tests.
Efficacy results are summarized in Tables 49 and 50.
Table 49: Efficacy Results for Patients with MSI-H/dMMR Cancer Endpoint KEYTRUDA
n=149NR = not reached Objective Response Rate ORR (95% CI) 39.6% (31.7, 47.9) Complete response rate 7.4% Partial response rate 32.2% Duration of Response Median in months (range) NR (1.6+, 22.7+) % with duration ≥6 months 78% Table 50: Response by Tumor Type Objective response rate DoR range N n (%) 95% CI (months) CR = complete response
PR = partial response
SD = stable disease
PD = progressive disease
NE = not evaluableCRC 90 32 (36%) (26%, 46%) (1.6+, 22.7+) Non-CRC 59 27 (46%) (33%, 59%) (1.9+, 22.1+) Endometrial cancer 14 5 (36%) (13%, 65%) (4.2+, 17.3+) Biliary cancer 11 3 (27%) (6%, 61%) (11.6+, 19.6+) Gastric or GE junction cancer 9 5 (56%) (21%, 86%) (5.8+, 22.1+) Pancreatic cancer 6 5 (83%) (36%, 100%) (2.6+, 9.2+) Small intestinal cancer 8 3 (38%) (9%, 76%) (1.9+, 9.1+) Breast cancer 2 PR, PR (7.6, 15.9) Prostate cancer 2 PR, SD 9.8+ Bladder cancer 1 NE Esophageal cancer 1 PR 18.2+ Sarcoma 1 PD Thyroid cancer 1 NE Retroperitoneal adenocarcinoma 1 PR 7.5+ Small cell lung cancer 1 CR 8.9+ Renal cell cancer 1 PD 14.9 Gastric Cancer
The efficacy of KEYTRUDA was investigated in KEYNOTE-059 (NCT02335411), a multicenter, non-randomized, open-label multi-cohort trial that enrolled 259 patients with gastric or gastroesophageal junction (GEJ) adenocarcinoma who progressed on at least 2 prior systemic treatments for advanced disease. Previous treatment must have included a fluoropyrimidine and platinum doublet. HER2/neu positive patients must have previously received treatment with approved HER2/neu-targeted therapy. Patients with active autoimmune disease or a medical condition that required immunosuppression or with clinical evidence of ascites by physical exam were ineligible. Patients received KEYTRUDA 200 mg every 3 weeks until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at least 4 weeks later with repeat imaging. Patients without disease progression were treated for up to 24 months. Assessment of tumor status was performed every 6 to 9 weeks. The major efficacy outcome measures were ORR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR, and DoR.
Among the 259 patients, 55% (n = 143) had tumors that expressed PD-L1 with a CPS ≥1 and microsatellite stable (MSS) tumor status or undetermined MSI or MMR status. PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx kit. The baseline characteristics of these 143 patients were: median age of 64 years, 47% age 65 or older; 77% male; 82% White and 11% Asian; and 43% ECOG PS of 0 and 57% ECOG PS of 1. Eighty-five percent had M1 disease and 7% had M0 disease. Fifty-one percent had two and 49% had three or more prior lines of therapy in the recurrent or metastatic setting.
For the 143 patients, the ORR was 13.3% (95% CI: 8.2, 20.0); 1.4% had a complete response and 11.9% had a partial response. Among the 19 responding patients, the DoR ranged from 2.8+ to 19.4+ months, with 11 patients (58%) having responses of 6 months or longer and 5 patients (26%) having responses of 12 months or longer.
Among the 259 patients enrolled in KEYNOTE-059, 7 (3%) had tumors that were determined to be MSI-H. An objective response was observed in 4 patients, including 1 complete response. The DoR ranged from 5.3+ to 14.1+ months.
14.10 Esophageal Cancer
KEYNOTE-181
The efficacy of KEYTRUDA was investigated in KEYNOTE-181 (NCT02564263), a multicenter, randomized, open-label, active-controlled trial that enrolled 628 patients with recurrent locally advanced or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced disease. Patients with HER2/neu positive esophageal cancer were required to have received treatment with approved HER2/neu targeted therapy. All patients were required to have tumor specimens for PD-L1 testing at a central laboratory; PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx kit. Patients with a history of non-infectious pneumonitis that required steroids or current pneumonitis, active autoimmune disease, or a medical condition that required immunosuppression were ineligible.
Patients were randomized (1:1) to receive either KEYTRUDA 200 mg every 3 weeks or investigator's choice of any of the following chemotherapy regimens, all given intravenously: paclitaxel 80-100 mg/m2 on Days 1, 8, and 15 of every 4-week cycle, docetaxel 75 mg/m2 every 3 weeks, or irinotecan 180 mg/m2 every 2 weeks. Randomization was stratified by tumor histology (esophageal squamous cell carcinoma [ESCC] vs. esophageal adenocarcinoma [EAC]/Siewert type I EAC of the gastroesophageal junction [GEJ]), and geographic region (Asia vs. ex-Asia). Treatment with KEYTRUDA or chemotherapy continued until unacceptable toxicity or disease progression. Patients randomized to KEYTRUDA were permitted to continue beyond the first RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined disease progression if clinically stable until the first radiographic evidence of disease progression was confirmed at least 4 weeks later with repeat imaging. Patients treated with KEYTRUDA without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks. The major efficacy outcome measure was OS evaluated in the following co-primary populations: patients with ESCC, patients with tumors expressing PD-L1 CPS ≥10, and all randomized patients. Additional efficacy outcome measures were PFS, ORR, and DoR, according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR.
A total of 628 patients were enrolled and randomized to KEYTRUDA (n=314) or investigator's treatment of choice (n=314). Of these 628 patients, 167 (27%) had ESCC that expressed PD-L1 with a CPS ≥10. Of these 167 patients, 85 patients were randomized to KEYTRUDA and 82 patients to investigator's treatment of choice [paclitaxel (n=50), docetaxel (n=19), or irinotecan (n=13)]. The baseline characteristics of these 167 patients were: median age of 65 years (range: 33 to 80), 51% age 65 or older; 84% male; 32% White and 68% Asian; 38% had an ECOG PS of 0 and 62% had an ECOG PS of 1. Ninety percent had M1 disease and 10% had M0 disease. Prior to enrollment, 99% of patients had received platinum-based treatment and 84% had also received treatment with a fluoropyrimidine. Thirty-three percent of patients received prior treatment with a taxane.
The observed OS hazard ratio was 0.77 (95% CI: 0.63, 0.96) in patients with ESCC, 0.70 (95% CI: 0.52, 0.94) in patients with tumors expressing PD-L1 CPS ≥10, and 0.89 (95% CI: 0.75, 1.05) in all randomized patients. On further examination in patients whose ESCC tumors expressed PD-L1 (CPS ≥10), an improvement in OS was observed among patients randomized to KEYTRUDA as compared with chemotherapy. Table 51 and Figure 12 summarize the key efficacy measures for KEYNOTE-181 for patients with ESCC CPS ≥10.
Table 51: Efficacy Results in Patients with Recurrent or Metastatic ESCC (CPS ≥10) in KEYNOTE-181 Endpoint KEYTRUDA
200 mg every 3 weeks
n=85Chemotherapy
n=82- * Based on the Cox regression model stratified by geographic region (Asia vs. ex-Asia)
OS Number (%) of patients with event 68 (80%) 72 (88%) Median in months (95% CI) 10.3 (7.0, 13.5) 6.7 (4.8, 8.6) Hazard ratio* (95% CI) 0.64 (0.46, 0.90) PFS Number (%) of patients with event 76 (89%) 76 (93%) Median in months (95% CI) 3.2 (2.1, 4.4) 2.3 (2.1, 3.4) Hazard ratio* (95% CI) 0.66 (0.48, 0.92) Objective Response Rate ORR (95% CI) 22 (14, 33) 7 (3, 15) Number (%) of complete responses 4 (5) 1 (1) Number (%) of partial responses 15 (18) 5 (6) Median duration of response in months (range) 9.3 (2.1+, 18.8+) 7.7 (4.3, 16.8+) Figure 12: Kaplan-Meier Curve for Overall Survival in KEYNOTE-181 (ESCC CPS ≥10) 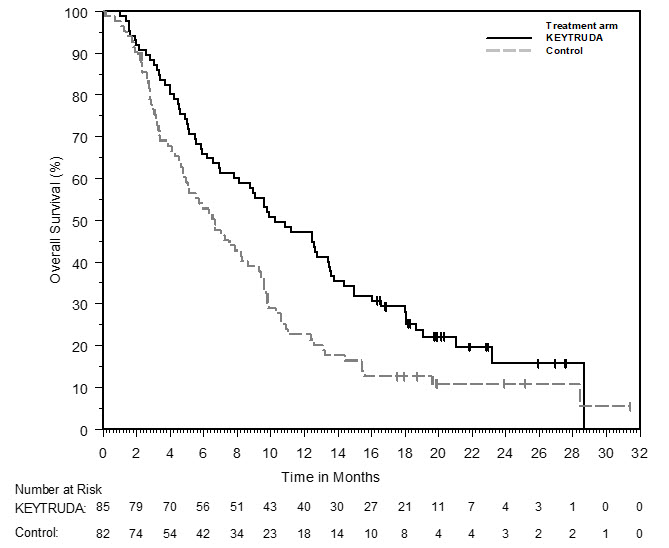
KEYNOTE-180
The efficacy of KEYTRUDA was investigated in KEYNOTE-180 (NCT02559687), a multicenter, non-randomized, open-label trial that enrolled 121 patients with locally advanced or metastatic esophageal cancer who progressed on or after at least 2 prior systemic treatments for advanced disease. With the exception of the number of prior lines of treatment, the eligibility criteria were similar to and the dosage regimen identical to KEYNOTE-181.
The major efficacy outcome measures were ORR and DoR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR.
Among the 121 patients enrolled, 29% (n=35) had ESCC that expressed PD-L1 CPS ≥10. The baseline characteristics of these 35 patients were: median age of 65 years (range: 47 to 81), 51% age 65 or older; 71% male; 26% White and 69% Asian; 40% had an ECOG PS of 0 and 60% had an ECOG PS of 1. One hundred percent had M1 disease.
The ORR in the 35 patients with ESCC expressing PD-L1 was 20% (95% CI: 8, 37). Among the 7 responding patients, the DoR ranged from 4.2 to 25.1+ months, with 5 patients (71%) having responses of 6 months or longer and 3 patients (57%) having responses of 12 months or longer.
14.11 Cervical Cancer
The efficacy of KEYTRUDA was investigated in 98 patients with recurrent or metastatic cervical cancer enrolled in a single cohort (Cohort E) in KEYNOTE-158 (NCT02628067), a multicenter, non-randomized, open-label, multi-cohort trial. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression. Patients received KEYTRUDA 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks for the first 12 months, and every 12 weeks thereafter. The major efficacy outcome measures were ORR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR, and DoR.
Among the 98 patients in Cohort E, 77 (79%) had tumors that expressed PD-L1 with a CPS ≥ 1 and received at least one line of chemotherapy in the metastatic setting. PD-L1 status was determined using the IHC 22C3 pharmDx kit. The baseline characteristics of these 77 patients were: median age of 45 years (range: 27 to 75); 81% White, 14% Asian, and 3% Black; 32% ECOG PS of 0 and 68% ECOG PS of 1; 92% had squamous cell carcinoma, 6% adenocarcinoma, and 1% adenosquamous histology; 95% had M1 disease and 5% had recurrent disease; and 35% had one and 65% had two or more prior lines of therapy in the recurrent or metastatic setting.
No responses were observed in patients whose tumors did not have PD-L1 expression (CPS <1). Efficacy results are summarized in Table 52 for patients with PD-L1 expression (CPS ≥1).
Table 52: Efficacy Results in Patients with Recurrent or Metastatic Cervical Cancer (CPS ≥1) in KEYNOTE-158 Endpoint KEYTRUDA
200 mg every 3 weeks
n=77*+ Denotes ongoing
NR = not reached- * Median follow-up time of 11.7 months (range 0.6 to 22.7 months)
- † Based on patients (n=11) with a response by independent review
Objective Response Rate ORR (95% CI) 14.3% (7.4, 24.1) Complete response rate 2.6% Partial response rate 11.7% Duration of Response Median in months (range) NR (4.1, 18.6+)† % with duration ≥6 months 91% 14.12 Hepatocellular Carcinoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-224 (NCT02702414), a single-arm, multicenter trial in 104 patients with HCC who had disease progression on or after sorafenib or were intolerant to sorafenib; had measurable disease; and Child-Pugh class A liver impairment. Patients with active autoimmune disease, greater than one etiology of hepatitis, a medical condition that required immunosuppression, or clinical evidence of ascites by physical exam were ineligible for the trial. Patients received KEYTRUDA 200 mg intravenously every 3 weeks until unacceptable toxicity, investigator-assessed confirmed disease progression (based on repeat scan at least 4 weeks from the initial scan showing progression), or completion of 24 months of KEYTRUDA. Assessment of tumor status was performed every 9 weeks. The major efficacy outcome measures were ORR and DoR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR.
The study population characteristics were: median age of 68 years, 67% age 65 or older; 83% male; 81% White and 14% Asian; and 61% ECOG PS of 0 and 39% ECOG PS of 1. Child-Pugh class and score were A5 for 72%, A6 for 22%, B7 for 5%, and B8 for 1% of patients. Twenty-one percent of the patients were HBV seropositive and 25% HCV seropositive. There were 9 patients (9%) who were seropositive for both HBV and HCV. For these 9 patients, all of the HBV cases and three of the HCV cases were inactive. Sixty-four percent (64%) of patients had extrahepatic disease, 17% had vascular invasion, and 9% had both. Thirty-eight percent (38%) of patients had alpha-fetoprotein (AFP) levels ≥400 mcg/L. All patients received prior sorafenib; of whom 20% were unable to tolerate sorafenib. No patient received more than one prior systemic therapy (sorafenib).
Efficacy results are summarized in Table 53.
Table 53: Efficacy Results in KEYNOTE-224 Endpoint KEYTRUDA
200 mg every 3 weeks
n=104- * Based on patients (n=18) with a confirmed response by independent review
BICR-Assessed Objective Response Rate (RECIST v1.1) ORR (95% CI)* 17% (11, 26) Complete response rate 1% Partial response rate 16% BICR-Assessed Duration of Response % with duration ≥6 months 89% % with duration ≥12 months 56% 14.13 Merkel Cell Carcinoma
The efficacy of KEYTRUDA was investigated in KEYNOTE-017 (NCT02267603), a multicenter, non-randomized, open-label trial that enrolled 50 patients with recurrent locally advanced or metastatic MCC who had not received prior systemic therapy for their advanced disease. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible.
Patients received KEYTRUDA 2 mg/kg every 3 weeks until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at least 4 weeks later with repeat imaging. Patients without disease progression were treated for up to 24 months. Assessment of tumor status was performed at 13 weeks followed by every 9 weeks for the first year and every 12 weeks thereafter. The major efficacy outcome measures were ORR and DoR as assessed by BICR per RECIST v1.1.
The study population characteristics were: median age of 71 years (range: 46 to 91), 80% age 65 or older; 68% male; 90% White; and 48% ECOG PS of 0 and 52% ECOG PS of 1. Fourteen percent had stage IIIB disease and 86% had stage IV. Eighty-four percent of patients had prior surgery and 70% had prior radiation therapy.
Efficacy results are summarized in Table 54.
Table 54: Efficacy Results in KEYNOTE-017 Endpoint KEYTRUDA
2 mg/kg every 3 weeks
n=50- * The median duration of response was not reached.
Objective Response Rate ORR (95% CI) 56% (41, 70) Complete response rate (95% CI) 24% (13, 38) Partial response rate (95% CI) 32% (20, 47) Duration of Response Range in months* 5.9-34.5+ Patients with duration ≥6 months, n (%) 27 (96%) Patients with duration ≥12 months, n (%) 15 (54%) 14.14 Renal Cell Carcinoma
The efficacy of KEYTRUDA in combination with axitinib was investigated in KEYNOTE-426 (NCT02853331), a randomized, multicenter, open-label trial conducted in 861 patients who had not received systemic therapy for advanced RCC. Patients were enrolled regardless of PD-L1 tumor expression status. Patients with active autoimmune disease requiring systemic immunosuppression within the last 2 years were ineligible. Randomization was stratified by International Metastatic RCC Database Consortium (IMDC) risk categories (favorable versus intermediate versus poor) and geographic region (North America versus Western Europe versus "Rest of the World").
Patients were randomized (1:1) to one of the following treatment arms:
- KEYTRUDA 200 mg intravenously every 3 weeks up to 24 months in combination with axitinib 5 mg orally, twice daily. Patients who tolerated axitinib 5 mg twice daily for 2 consecutive cycles (6 weeks) could increase to 7 mg and then subsequently to 10 mg twice daily. Axitinib could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg orally, once daily for 4 weeks and then off treatment for 2 weeks.
Treatment with KEYTRUDA and axitinib continued until RECIST v1.1-defined progression of disease or unacceptable toxicity. Administration of KEYTRUDA and axitinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, after randomization at Week 12, then every 6 weeks thereafter until Week 54, and then every 12 weeks thereafter.
The study population characteristics were: median age of 62 years (range: 26 to 90); 38% age 65 or older; 73% male; 79% White and 16% Asian; 19% and 80% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; and patient distribution by IMDC risk categories was 31% favorable, 56% intermediate and 13% poor.
The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures included ORR, as assessed by BICR. A statistically significant improvement in OS was demonstrated at the pre-specified interim analysis in patients randomized to KEYTRUDA in combination with axitinib compared with sunitinib. The trial also demonstrated statistically significant improvements in PFS and ORR. Table 55 and Figure 13 summarize the efficacy results for KEYNOTE-426. The median follow-up time was 12.8 months (range 0.1 to 22.0 months). Consistent results were observed across pre-specified subgroups, IMDC risk categories and PD-L1 tumor expression status.
Table 55: Efficacy Results in KEYNOTE-426 Endpoint KEYTRUDA
200 mg every 3 weeks and AxitinibSunitinib n=432 n=429 NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on stratified log-rank test
- ‡ p-Value (one-sided) is compared with the allocated alpha of 0.0001 for this interim analysis (with 39% of the planned number of events for final analysis).
- § p-Value (one-sided) is compared with the allocated alpha of 0.0013 for this interim analysis (with 81% of the planned number of events for final analysis).
- ¶ Based on Miettinen and Nurminen method stratified by IMDC risk group and geographic region
OS Number of patients with event (%) 59 (14%) 97 (23%) Median in months (95% CI) NR (NR, NR) NR (NR, NR) Hazard ratio* (95% CI) 0.53 (0.38, 0.74) p-Value† <0.0001‡ 12-month OS rate 90% (86, 92) 78% (74, 82) PFS Number of patients with event (%) 183 (42%) 213 (50%) Median in months (95% CI) 15.1 (12.6, 17.7) 11.0 (8.7, 12.5) Hazard ratio* (95% CI) 0.69 (0.56, 0.84) p-Value† 0.0001§ ORR Overall confirmed response rate (95% CI) 59% (54, 64) 36% (31, 40) Complete response rate 6% 2% Partial response rate 53% 34% p-Value¶ <0.0001 Figure 13: Kaplan-Meier Curve for Overall Survival in KEYNOTE-426 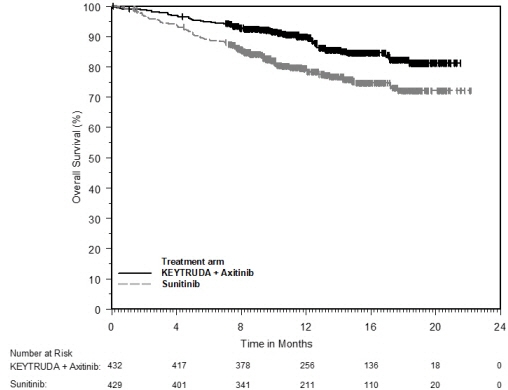
14.15 Endometrial Carcinoma
The efficacy of KEYTRUDA in combination with lenvatinib was investigated in KEYNOTE-146 (NCT02501096), a single-arm, multicenter, open-label, multi-cohort trial that enrolled 108 patients with metastatic endometrial carcinoma that had progressed following at least one prior systemic therapy in any setting. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible. Patients were treated with KEYTRUDA 200 mg intravenously every 3 weeks in combination with lenvatinib 20 mg orally once daily until unacceptable toxicity or disease progression as determined by the investigator. The major efficacy outcome measures were ORR and DoR as assessed by BICR using RECIST 1.1.
Administration of KEYTRUDA and lenvatinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered by the investigator to be deriving clinical benefit. KEYTRUDA dosing was continued for a maximum of 24 months; however, treatment with lenvatinib could be continued beyond 24 months. Assessment of tumor status was performed at baseline and then every 6 weeks until week 24, followed by every 9 weeks thereafter.
Among the 108 patients, 87% (n=94) had tumors that were not MSI-H or dMMR, 10% (n=11) had tumors that were MSI-H or dMMR, and in 3% (n=3) the status was not known. Tumor MSI status was determined using a polymerase chain reaction (PCR) test. Tumor MMR status was determined using an IHC test. The baseline characteristics of the 94 patients with tumors that were not MSI-H or dMMR were: median age of 66 years, 62% age 65 or older; 86% White, 6% Black, 4% Asian, and 3% other races; and ECOG PS of 0 (52%) or 1 (48%). All 94 of these patients received prior systemic therapy for endometrial carcinoma: 51% had one, 38% had two, and 11% had three or more prior systemic therapies.
Efficacy results are summarized in Table 56.
Table 56: Efficacy Results in KEYNOTE-146 Endpoint KEYTRUDA
200 mg every 3 weeks with lenvatinib
n=94*+ Denotes ongoing
NR = not reached- * Median follow-up time of 18.7 months
- † Based on patients (n=36) with a response by independent review
Objective Response Rate ORR (95% CI) 38.3% (29, 49) Complete response rate 10.6% Partial response rate 27.7% Response duration Median in months (range) NR (1.2+, 33.1+)† % with duration ≥6 months 69% -
16 HOW SUPPLIED/STORAGE AND HANDLING
KEYTRUDA injection (clear to slightly opalescent, colorless to slightly yellow solution):
Carton containing one 100 mg/4 mL (25 mg/mL), single-dose vial (NDC: 0006-3026-02)
Carton containing two 100 mg/4 mL (25 mg/mL), single-dose vials (NDC: 0006-3026-04)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
- Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of KEYTRUDA. These reactions may include:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for new or worsening cough, chest pain, or shortness of breath [see Warnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea or severe abdominal pain [see Warnings and Precautions (5.2)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, or easy bruising or bleeding [see Warnings and Precautions (5.3)].
- Adrenal Insufficiency: Advise patients to contact their healthcare provider immediately for extreme weakness, dizziness, or fainting [see Warnings and Precautions (5.4)].
- Hypophysitis: Advise patients to contact their healthcare provider immediately for persistent or unusual headache, extreme weakness, dizziness or fainting, or vision changes [see Warnings and Precautions (5.4)].
- Hyperthyroidism and Hypothyroidism: Advise patients to contact their healthcare provider immediately for signs or symptoms of hyperthyroidism and hypothyroidism [see Warnings and Precautions (5.4)].
- Type 1 Diabetes Mellitus: Advise patients to contact their healthcare provider immediately for signs or symptoms of type 1 diabetes [see Warnings and Precautions (5.4)].
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis [see Warnings and Precautions (5.5)].
- Severe skin reactions: Advise patients to contact their healthcare provider immediately for any signs or symptoms of severe skin reactions, SJS or TEN [see Warnings and Precautions (5.6)].
- Other immune-mediated adverse reactions:
- Advise patients that immune-mediated adverse reactions can occur and may involve any organ system, and to contact their healthcare provider immediately for any new signs or symptoms [see Warnings and Precautions (5.7)].
- Advise patients of the risk of solid organ transplant rejection and to contact their healthcare provider immediately for signs or symptoms of organ transplant rejection [see Warnings and Precautions (5.7)].
Infusion-Related Reactions
- Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.8)].
Complications of Allogeneic HSCT
- Advise patients of the risk of post-allogeneic hematopoietic stem cell transplantation complications [see Warnings and Precautions (5.9)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.11), Use in Specific Populations (8.1, 8.3)].
- Advise females of reproductive potential to use effective contraception during treatment with KEYTRUDA and for 4 months after the last dose [see Warnings and Precautions (5.11), Use in Specific Populations (8.1, 8.3)].
Lactation
- Advise women not to breastfeed during treatment with KEYTRUDA and for 4 months after the final dose [see Use in Specific Populations (8.2)].
Laboratory Tests
- Advise patients of the importance of keeping scheduled appointments for blood work or other laboratory tests [see Warnings and Precautions (5.3, 5.4, 5.5)].
- Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of KEYTRUDA. These reactions may include:
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
U.S. License No. 0002For KEYTRUDA injection, at:
MSD Ireland (Carlow)
County Carlow, IrelandFor patent information: www.merck.com/product/patent/home.html
The trademarks depicted herein are owned by their respective companies.
Copyright © 2014-2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.uspi-mk3475-iv-2001r032
-
MEDICATION GUIDE
MEDICATION GUIDE KEYTRUDA® (key-true-duh)
(pembrolizumab)
injectionThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: January 2020 What is the most important information I should know about KEYTRUDA?
KEYTRUDA is a medicine that may treat certain cancers by working with your immune system. KEYTRUDA can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. These problems may happen anytime during treatment or even after your treatment has ended.
Call or see your doctor right away if you develop any symptoms of the following problems or these symptoms get worse:
Lung problems (pneumonitis). Symptoms of pneumonitis may include:- shortness of breath
- chest pain
- new or worse cough
- diarrhea or more bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
- yellowing of your skin or the whites of your eyes
- nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine
- bleeding or bruising more easily than normal
- rapid heart beat
- weight loss or weight gain
- increased sweating
- feeling more hungry or thirsty
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- muscle aches
- feeling very weak
- dizziness or fainting
- headaches that will not go away or unusual headache
- change in the amount or color of your urine
- rash
- itching
- blisters, peeling or skin sores
- painful sores or ulcers in your mouth or in your nose, throat, or genital area
- changes in eyesight
- severe or persistent muscle or joint pains
- severe muscle weakness
- low red blood cells (anemia)
- swollen lymph nodes, rash or tender lumps on skin, cough, shortness of breath, vision changes, or eye pain (sarcoidosis)
- confusion, fever, muscle weakness, balance problems, nausea, vomiting, stiff neck, memory problems, or seizures (encephalitis)
- shortness of breath, irregular heartbeat, feeling tired, or chest pain (myocarditis)
- chills or shaking
- shortness of breath or wheezing
- itching or rash
- flushing
- dizziness
- fever
- feeling like passing out
Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be severe and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with KEYTRUDA. Your doctor will monitor you for the following signs and symptoms: skin rash, liver inflammation, stomach-area (abdominal) pain, and diarrhea.
Getting medical treatment right away may help keep these problems from becoming more serious.
Your doctor will check you for these problems during treatment with KEYTRUDA. Your doctor may treat you with corticosteroid or hormone replacement medicines. Your doctor may also need to delay or completely stop treatment with KEYTRUDA, if you have severe side effects.What is KEYTRUDA?
KEYTRUDA is a prescription medicine used to treat:- a kind of skin cancer called melanoma. KEYTRUDA may be used:
- when your melanoma has spread or cannot be removed by surgery (advanced melanoma), or
- to help prevent melanoma from coming back after it and lymph nodes that contain cancer have been removed by surgery.
- a kind of lung cancer called non-small cell lung cancer (NSCLC).
- KEYTRUDA may be used with the chemotherapy medicines pemetrexed and a platinum as your first treatment when your lung cancer:
- has spread (advanced NSCLC), and
- is a type called "nonsquamous", and
- your tumor does not have an abnormal "EGFR" or "ALK" gene.
- KEYTRUDA may be used with the chemotherapy medicines carboplatin and either paclitaxel or paclitaxel protein-bound as your first treatment when your lung cancer:
- has spread (advanced NSCLC), and
- is a type called "squamous".
- KEYTRUDA may be used alone as your first treatment when your lung cancer:
- has not spread outside your chest (stage III) and you cannot have surgery or chemotherapy with radiation or
- your NSCLC has spread to other areas of your body (advanced NSCLC), and
- your tumor tests positive for "PD-L1", and
- does not have an abnormal "EGFR" or "ALK" gene.
- KEYTRUDA may also be used alone when:
- you have received chemotherapy that contains platinum to treat your advanced NSCLC, and it did not work or it is no longer working, and
- your tumor tests positive for "PD-L1", and
- if your tumor has an abnormal "EGFR" or "ALK" gene, you have also received an EGFR or ALK inhibitor medicine and it did not work or is no longer working.
- KEYTRUDA may be used with the chemotherapy medicines pemetrexed and a platinum as your first treatment when your lung cancer:
- a kind of lung cancer called small cell lung cancer (SCLC). KEYTRUDA may be used when your lung cancer:
- has spread (advanced SCLC), and
- you have received 2 or more types of chemotherapy, including one that contains platinum, and it did not work or is no longer working.
- a kind of cancer called head and neck squamous cell cancer (HNSCC).
- KEYTRUDA may be used with the chemotherapy medicines fluorouracil and a platinum as your first treatment when your head and neck cancer has spread or returned and cannot be removed by surgery.
- KEYTRUDA may be used alone as your first treatment when your head and neck cancer:
- has spread or returned and cannot be removed by surgery, and
- your tumor tests positive for "PD-L1".
- KEYTRUDA may be used alone when your head and neck cancer:
- has spread or returned, and
- you have received chemotherapy that contains platinum and it did not work or is no longer working.
- a kind of cancer called classical Hodgkin lymphoma (cHL) in adults and children when:
- you have tried a treatment and it did not work or
- your cHL has returned after you received 3 or more types of treatment.
- a kind of cancer called primary mediastinal B-cell lymphoma (PMBCL) in adults and children when:
- you have tried a treatment and it did not work or
- your PMBCL has returned after you received 2 or more types of treatment.
- a kind of bladder and urinary tract cancer called urothelial carcinoma.
- KEYTRUDA may be used when your cancer has not spread to nearby tissue in the bladder, but is at high-risk for spreading (high-risk non-muscle-invasive bladder cancer [NMIBC]) when:
- your tumor is a type called "carcinoma in situ" (CIS), and
- you have tried treatment with Bacillus Calmette-Guerin (BCG) and it did not work, and
- you are not able to or have decided not to have surgery to remove your bladder.
- KEYTRUDA may be used when your cancer has not spread to nearby tissue in the bladder, but is at high-risk for spreading (high-risk non-muscle-invasive bladder cancer [NMIBC]) when:
- KEYTRUDA may be used when your bladder or urinary tract cancer:
- has spread or cannot be removed by surgery (advanced urothelial cancer) and,
- you are not able to receive chemotherapy that contains a medicine called cisplatin, and your tumor tests positive for "PD-L1", or
- you are not able to receive a medicine called cisplatin or carboplatin, or
- you have received chemotherapy that contains platinum, and it did not work or is no longer working.
- a kind of cancer that is shown by a laboratory test to be a microsatellite instability-high (MSI-H) or a mismatch repair deficient (dMMR) solid tumor. KEYTRUDA may be used in adults and children to treat:
- cancer that has spread or cannot be removed by surgery (advanced cancer), and
- has progressed following treatment, and you have no satisfactory treatment options, or
- you have colon or rectal cancer, and you have received chemotherapy with fluoropyrimidine, oxaliplatin, and irinotecan but it did not work or is no longer working.
- a kind of stomach cancer called gastric or gastroesophageal junction (GEJ) adenocarcinoma that tests positive for "PD-L1." KEYTRUDA may be used when your stomach cancer:
- has returned or spread (advanced gastric cancer), and
- you have received 2 or more types of chemotherapy including fluoropyrimidine and chemotherapy that contains platinum, and it did not work or is no longer working, and
- if your tumor has an abnormal "HER2/neu" gene, you also received a HER2/neu-targeted medicine and it did not work or is no longer working.
- a kind of cancer called squamous cell carcinoma of the esophagus. KEYTRUDA may be used when:
- your cancer has returned or spread (advanced esophageal cancer), and
- your tumor tests positive for "PD-L1" and you have received one or more types of treatment and it did not work or is no longer working.
- a kind of cancer called cervical cancer that tests positive for "PD-L1." KEYTRUDA may be used when your cervical cancer:
- has returned, or has spread or cannot be removed by surgery (advanced cervical cancer), and
- you have received chemotherapy, and it did not work or is no longer working.
- a kind of liver cancer called hepatocellular carcinoma, after you have received the medicine sorafenib.
- a kind of skin cancer called Merkel cell carcinoma (MCC) in adults and children. KEYTRUDA may be used to treat your skin cancer when it has spread or returned.
- a kind of kidney cancer called renal cell carcinoma (RCC). KEYTRUDA may be used with the medicine axitinib as your first treatment when your kidney cancer has spread or cannot be removed by surgery (advanced RCC).
- a kind of uterine cancer called endometrial carcinoma. KEYTRUDA may be used with the medicine lenvatinib:
- when your tumors are not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and
- you have received anti-cancer treatment, and it did not work or is no longer working, and
- your cancer cannot be removed by surgery or radiation (advanced endometrial carcinoma).
What should I tell my doctor before receiving KEYTRUDA?
Before you receive KEYTRUDA, tell your doctor if you:- have immune system problems such as Crohn's disease, ulcerative colitis, or lupus
- have received an organ transplant, such as a kidney or liver
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have lung or breathing problems
- have liver problems
- have any other medical problems
- are pregnant or plan to become pregnant
- KEYTRUDA can harm your unborn baby.
- Your doctor will give you a pregnancy test before you start treatment with KEYTRUDA.
- You should use an effective method of birth control during and for at least 4 months after the final dose of KEYTRUDA. Talk to your doctor about birth control methods that you can use during this time.
- Tell your doctor right away if you think you may be pregnant or if you become pregnant during treatment with KEYTRUDA.
- are breastfeeding or plan to breastfeed.
- It is not known if KEYTRUDA passes into your breast milk.
- Do not breastfeed during treatment with KEYTRUDA and for 4 months after your final dose of KEYTRUDA.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.How will I receive KEYTRUDA? - Your doctor will give you KEYTRUDA into your vein through an intravenous (IV) line over 30 minutes.
- KEYTRUDA is usually given every 3 weeks.
- Your doctor will decide how many treatments you need.
- Your doctor will do blood tests to check you for side effects.
- If you miss any appointments, call your doctor as soon as possible to reschedule your appointment.
What are the possible side effects of KEYTRUDA?
KEYTRUDA can cause serious side effects. See "What is the most important information I should know about KEYTRUDA?"
Common side effects of KEYTRUDA when used alone include: feeling tired, pain, including pain in muscles, bones or joints and stomach-area (abdominal) pain, decreased appetite, itching, diarrhea, nausea, rash, fever, cough, shortness of breath, and constipation.
Common side effects of KEYTRUDA when given with certain chemotherapy medicines include: feeling tired or weak, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, trouble breathing, fever, hair loss, inflammation of the nerves that may cause pain, weakness, and paralysis in the arms and legs, swelling of the lining of the mouth, nose, eyes, throat, intestines, or vagina, and mouth sores.
Common side effects of KEYTRUDA when given with axitinib include: diarrhea, feeling tired or weak, high blood pressure, liver problems, low levels of thyroid hormone, decreased appetite, blisters or rash on the palms of your hands and soles of your feet, nausea, mouth sores or swelling of the lining of the mouth, nose, eyes, throat, intestines, or vagina, hoarseness, rash, cough, and constipation.
Common side effects of KEYTRUDA when given with lenvatinib include: feeling tired, high blood pressure, joint and muscle pain, diarrhea, decreased appetite, low levels of thyroid hormone, nausea, mouth sores, vomiting, weight loss, stomach-area (abdominal) pain, headache, constipation, urinary tract infection, hoarseness, bleeding, low magnesium level, blisters or rash on the palms of your hands and soles of your feet, shortness of breath, cough, and rash.
In children, feeling tired, vomiting and stomach-area (abdominal) pain, and increased levels of liver enzymes and decreased levels of salt (sodium) in the blood are more common than in adults.
These are not all the possible side effects of KEYTRUDA. For more information, ask your doctor or pharmacist.
Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of KEYTRUDA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about KEYTRUDA, talk with your doctor. You can ask your doctor or nurse for information about KEYTRUDA that is written for healthcare professionals. For more information, go to www.keytruda.com.What are the ingredients in KEYTRUDA?
Active ingredient: pembrolizumab
Inactive ingredients:
KEYTRUDA injection: L-histidine, polysorbate 80, sucrose, and Water for Injection, USP.Manufactured by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAFor KEYTRUDA injection, at:
MSD Ireland (Carlow), County Carlow, Ireland
U.S. License No. 0002
For patent information: www.merck.com/product/patent/home.html
Copyright © 2014-2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
usmg-mk3475-iv-2001r029 -
PRINCIPAL DISPLAY PANEL - 50 mg Vial Carton
NDC: 0006-3029-02
Keytruda®
(pembrolizumab)
for Injection50 mg / vial
For Intravenous Infusion Only
Dispense the enclosed Medication Guide to each patient.
Sterile lyophilized powder must be reconstituted with Sterile Water for
Injection, USP. Reconstituted solution requires further dilution prior
to administration.Rx only
Single-dose vial. Discard unused portion.
-
PRINCIPAL DISPLAY PANEL - 100 mg/4 mL Vial Carton
NDC: 0006-3026-02
Keytruda®
(pembrolizumab)
Injection100 mg/4 mL
(25 mg/mL)For Intravenous Infusion Only
Dispense the enclosed Medication Guide to each patient.
Requires dilution prior to administration.
Rx only
Single-dose vial. Discard unused portion.
-
INGREDIENTS AND APPEARANCE
KEYTRUDA
pembrolizumab injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-3029 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pembrolizumab (UNII: DPT0O3T46P) (pembrolizumab - UNII:DPT0O3T46P) pembrolizumab 50 mg in 2 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 3.1 mg in 2 mL SUCROSE (UNII: C151H8M554) 140 mg in 2 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.4 mg in 2 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-3029-02 1 in 1 CARTON 09/04/2014 12/21/2015 1 NDC: 0006-3029-01 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125514 09/04/2014 12/21/2015 KEYTRUDA
pembrolizumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-3026 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pembrolizumab (UNII: DPT0O3T46P) (pembrolizumab - UNII:DPT0O3T46P) pembrolizumab 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 1.55 mg in 1 mL SUCROSE (UNII: C151H8M554) 70 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-3026-02 1 in 1 CARTON 01/15/2015 1 NDC: 0006-3026-01 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0006-3026-04 2 in 1 CARTON 08/01/2019 2 NDC: 0006-3026-01 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125514 01/15/2015 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Merck Sharp & Dohme Corp. 101740835 PACK(0006-3029, 0006-3026) , LABEL(0006-3029, 0006-3026) Establishment Name Address ID/FEI Business Operations MSD International GmbH 985655572 MANUFACTURE(0006-3029) , ANALYSIS(0006-3029, 0006-3026) Establishment Name Address ID/FEI Business Operations MSD Ireland (Carlow) 985084764 MANUFACTURE(0006-3026) , ANALYSIS(0006-3026) Establishment Name Address ID/FEI Business Operations N.V. Organon 404467722 ANALYSIS(0006-3029, 0006-3026) Establishment Name Address ID/FEI Business Operations Covance Laboratories 213137276 ANALYSIS(0006-3029, 0006-3026)
Trademark Results [KEYTRUDA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KEYTRUDA 86177261 not registered Dead/Abandoned |
Merck Sharp & Dohme Corp. 2014-01-28 |
 KEYTRUDA 86177260 4804821 Live/Registered |
Merck Sharp & Dohme Corp. 2014-01-28 |
 KEYTRUDA 86177257 not registered Dead/Abandoned |
Merck Sharp & Dohme Corp. 2014-01-28 |
 KEYTRUDA 86147051 not registered Dead/Abandoned |
Merck Sharp & Dohme Corp. 2013-12-18 |
 KEYTRUDA 86147047 4804749 Live/Registered |
Merck Sharp & Dohme Corp. 2013-12-18 |
 KEYTRUDA 86147045 not registered Dead/Abandoned |
Merck Sharp & Dohme Corp. 2013-12-18 |
 KEYTRUDA 79112065 4263031 Live/Registered |
Merck Sharp & Dohme Corp. 2011-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.