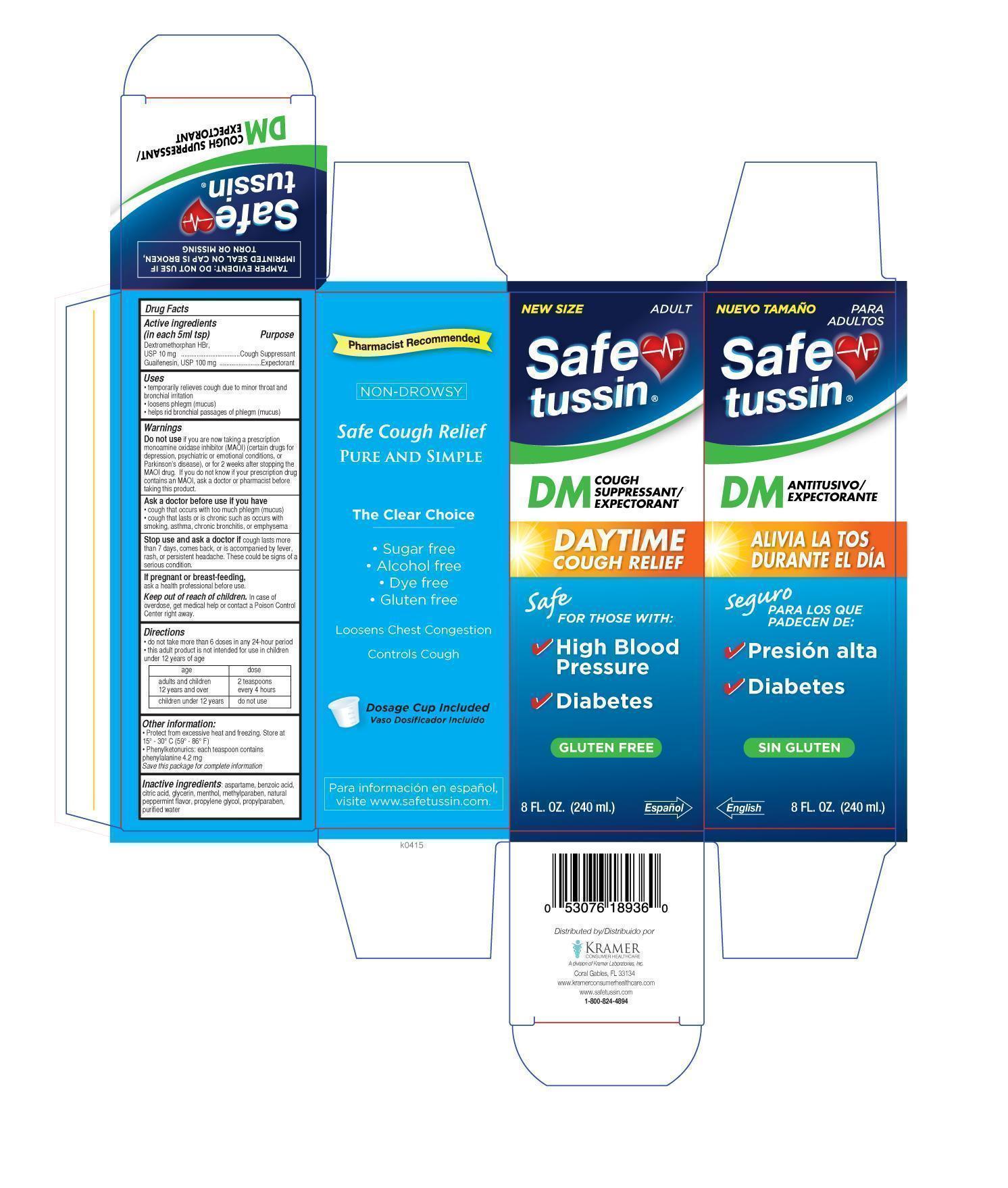
SAFETUSSIN DM- dextromethorpan guaifenesin liquid
Safetussin by
Drug Labeling and Warnings
Safetussin by is a Otc medication manufactured, distributed, or labeled by Kramer Laboratories, Denison Pharmecuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
WARNINGS
Do not use
If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.Ask a doctor before use if you have
cough that occurs with too much phlegm (mucus)
cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysemaStop use and ask a doctor if
cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SAFETUSSIN DM
dextromethorpan guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55505-189 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (Dextromethorphan - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength Aspartame (UNII: Z0H242BBR1) BENZOIC ACID (UNII: 8SKN0B0MIM) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) methylparaben (UNII: A2I8C7HI9T) MINT (UNII: FV98Z8GITP) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) Water (UNII: 059QF0KO0R) MENTHOL (UNII: L7T10EIP3A) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55505-189-36 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/01/2015 Labeler - Kramer Laboratories (122720675) Establishment Name Address ID/FEI Business Operations Denison Pharmecuticals 001207208 manufacture(55505-189)
Trademark Results [Safetussin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SAFETUSSIN 78592800 3068103 Live/Registered |
Kramer Laboratories, Inc. 2005-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.