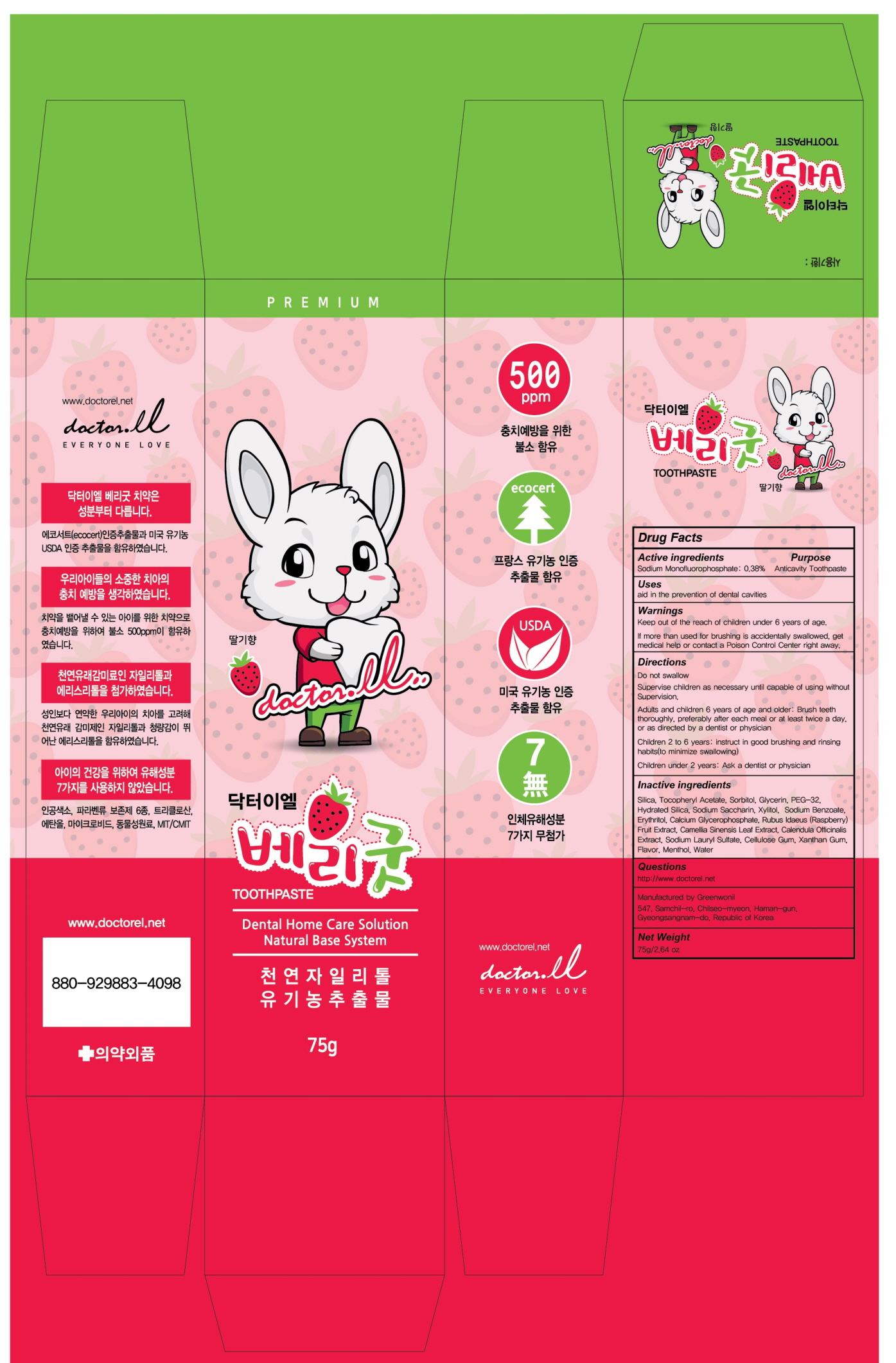
DR.EL Very Good Tooth by Dr. EL CO., LTD. / Green Wonil Co., Ltd
DR.EL Very Good Tooth by
Drug Labeling and Warnings
DR.EL Very Good Tooth by is a Otc medication manufactured, distributed, or labeled by Dr. EL CO., LTD., Green Wonil Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DR.EL VERY GOOD TOOTH- silica, sodiuoroum monoflphosphate, tocopheryl acetate, hydrated silica paste, dentifrice
Dr. EL CO., LTD.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Supervise children as necessary until capable of using without Supervision.
Adults and children 6 years of age and older: Bursh teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician
Children 2 to 6 years: Use only a pea sized amount and supervise child’s brushing and rinsing (to minimize swallpwing)
Children under 2 years: Ask a dentist or physician
Keep out of the reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away
Silica, Tocopheryl Acetate, Sorbitol, Glycerin, PEG-32, Hydrated Silica, Sodium Saccharin, Xylitol, Sodium Benzoate, Erythritol, Calcium Glycerophosphate, Rubus Idaeus (Raspberry) Fruit Extract, Camellia Sinensis Leaf Extract, Calendula Officinalis Extract, Sodium Lauryl Sulfate, Cellulose Gum, Xanthan Gum, Flavor, Menthol, Water
| DR.EL VERY GOOD TOOTH
silica, sodiuoroum monoflphosphate, tocopheryl acetate, hydrated silica paste, dentifrice |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Dr. EL CO., LTD. (694771074) |
| Registrant - Dr. EL CO., LTD. (694771074) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Green Wonil Co., Ltd | 688442099 | manufacture(72440-101) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr. EL CO., LTD. | 694771074 | label(72440-101) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.